4 - Membranes
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
Functions of Membrane Proteins
Transport
Receptors for signal transduction
Attachment to cytoskeleton and extracellular matrix
Enzymatic activity
Intercellular joining
Cell-cell recognition
Types of Membrane Transport
Plasma membranes selectively permeable
Some molecules pass through easily; some do not
Passive processes
No cellular energy (ATP) required
Substance moves down its concentration gradient
Active processes
Energy (ATP) required
Occurs only in living cell membranes
Types of Passive Transport
Diffusion
Facilitated transport
Osmosis
Diffusion
Passive process
Describes the spread of particles (which can also be atoms, molecules) through random motion from regions of higher concentration to regions of lower concentration
Collisions cause molecules to move down or with their concentration gradient
Difference in concentration between two areas
Diffusion can still occur when there is no concentration gradient (but there will be no net flux)
Driven by decrease in Gibbs free energy
Speed influenced by molecule size and temperature
Macroscopic theory of diffusion
Fick's first law of diffusion
Fick’s second law of diffusion
Fick's first law of diffusion
Net flux is proportional to the spatial gradient of the concentration function
Proposed in an analogy to Fourier’s law of heat transfer
J = -D(dC/dx)
J = Diffusion flux, amt of substance per unit area per unit time
D = Diffusion coefficient, length/time
C = Concentration, amount of substance per unit volume
x = position, length
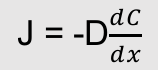
What is this equation
Fick’s first law of diffusion
J = Diffusion flux, amt of substance per unit area per unit time
D = Diffusion coefficient, length/time
C = Concentration, amount of substance per unit volume
x = position, length
Note: I do not think we need the equations memorized just be familiar
Fick’s second law of diffusion
The time rate of change in concentration is proportional to the curvature of the concentration function
Follows from continuity equation and Fick’s first law
Can be derived from the one-dimensional random walk
Predicts how diffusion causes the concentration to change with time
dc/dt = D * d2C/dx2
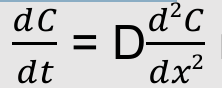
What is this equation
Fick’s second law of diffusion
shows time dependency
Note: I do not think we need the equations memorized just be familiar
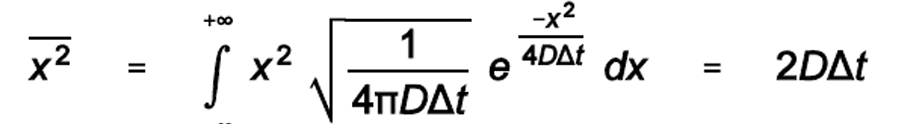
What is this equation?
The relationship between distance and time for diffusion
The time for one-dimensional diffusion increases as the square of the distance
Derived from the variance of the Gaussian distribution
2D diffusion: Variance is 4DΔt
3D diffusion (molecule across a cell): Variance is 6DΔt
For distances smaller than a cell (0-10 µm), diffusion takes less than a ms to a few ms
For larger distances (diameter of a muscle cell, 40-100 µm), diffusion can take several seconds
Liquid diffusion coefficient
Stokes showed that for a spherical particle, the drag force is related to size and solvent viscosity
Liquid diffusion coefficients from the Stokes Einstein equation
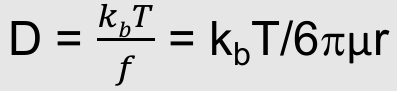
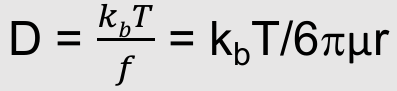
What is this equation:
Liquid diffusion coefficients from the Stokes
Where:
f = Friction coefficient of the solute
kb = Boltzmann’s constant
µ = Solvent viscosity
r = Solute radius
Permeability
aka: filtration
the rate of flow of a liquid or gas through a porous material
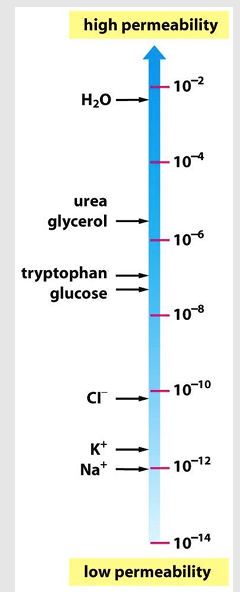
Diffusion
the passive movement of molecules or particles along a concentration gradient
Permeability depends on
diffusion coefficient (D)
P increases when D increases
Membrane thickness (Δx)
P decreases when Δx increase
Partition coefficient (K)
P increases when K increases
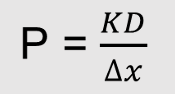
Diffusion across the lipid bilayer
Any molecule will eventually diffuse across a protein-free lipid bilayer down a concentration gradient (might just take a while)
The rate of diffusion depends on the size of the molecule and its hydrophobicity
Small nonpolar molecules (O2, CO2) diffuse rapidly
Small polar molecule (water, urea) diffuse slowly
Lipid bilayer is highly impermeable to charged ions
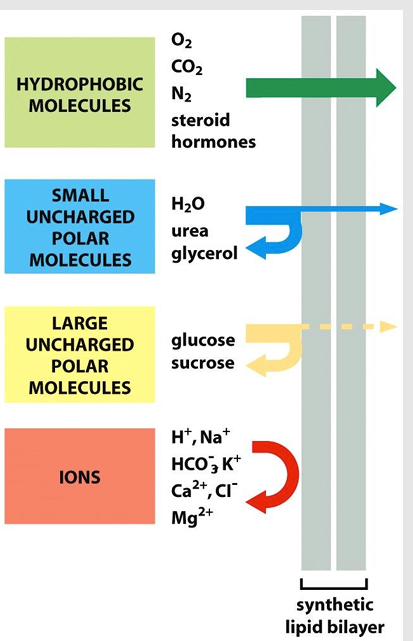
Transport across the lipid bilayer
Membrane transport proteins are needed to allow essential molecules to pass through the lipid bilayer
Ions, sugars, amino acids, nucleotides, cell metabolites
Transporters
Channels
Transporters
Bind a specific solute and undergo conformational changes to transfer the solute across the membrane
embedded in lipid layer
Channels
Form aqueous pores that extend across the lipid bilayer, example: aquaporins
Much faster transport than transport via proteins
Types of diffusion
simple diffusion
facilitated diffusion
Simple diffusion
Nonpolar lipid-soluble (hydrophobic) substances diffuse directly through phospholipid bilayer
E.g., oxygen, carbon dioxide, fat-soluble vitamins
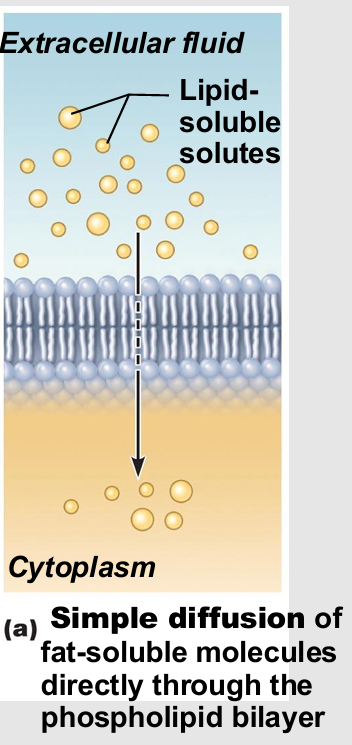
Facilitated Diffusion
Certain lipophobic molecules (e.g., glucose, amino acids, and ions) transported passively by
Binding to protein carriers
Moving through water-filled channels
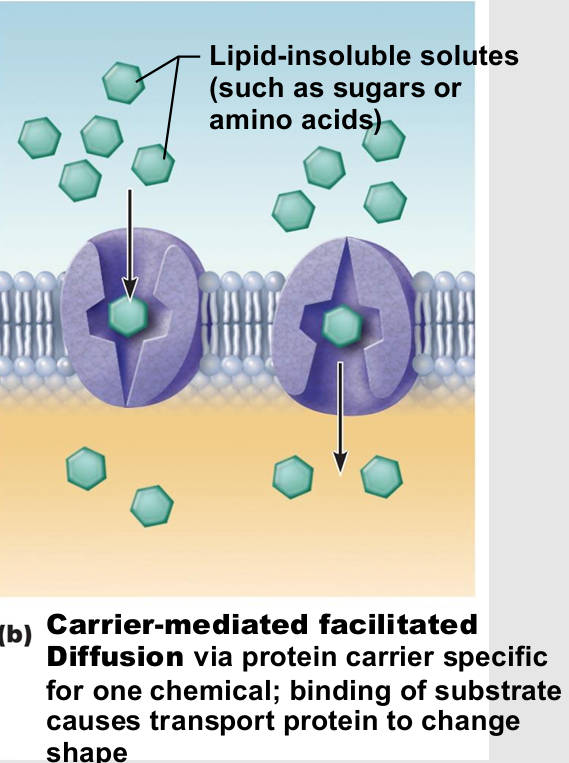
Carrier-mediated facilitated diffusion
passive therefore no energy
Transmembrane integral proteins are carriers
Transport specific polar molecules (e.g., sugars and amino acids) too large for channels
Binding of substrate causes shape change in carrier then passage across membrane
Limited by number of carriers present
Carriers saturated when all engaged
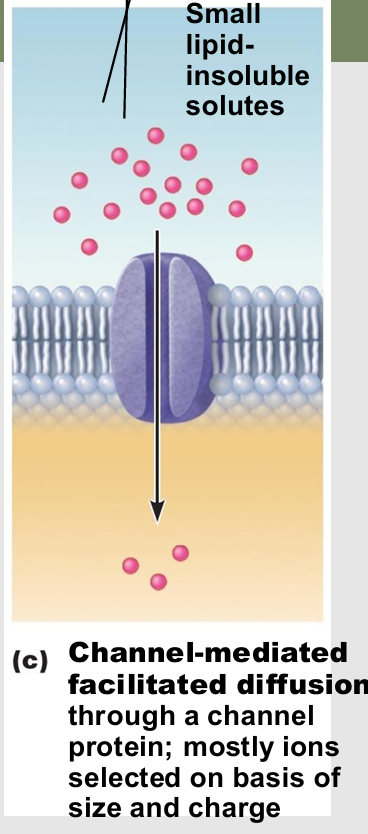
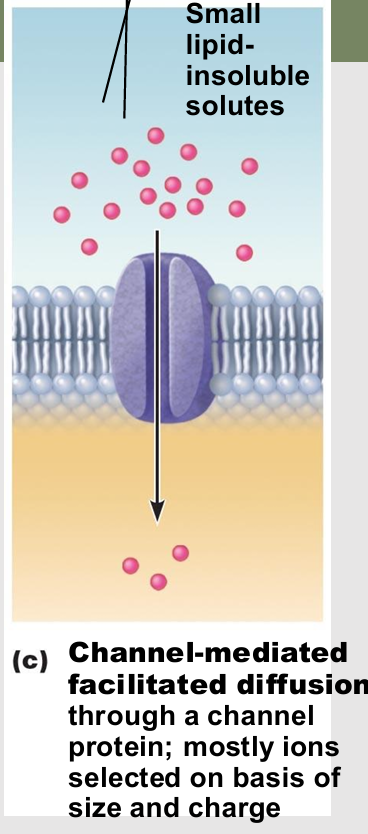
Channel-mediated facilitated diffusion
Aqueous channels formed by transmembrane proteins
Selectively transport ions or water
types
leakage channels
gated channels
Leakage channels
types of channel in Channel-mediated facilitated diffusion
always open
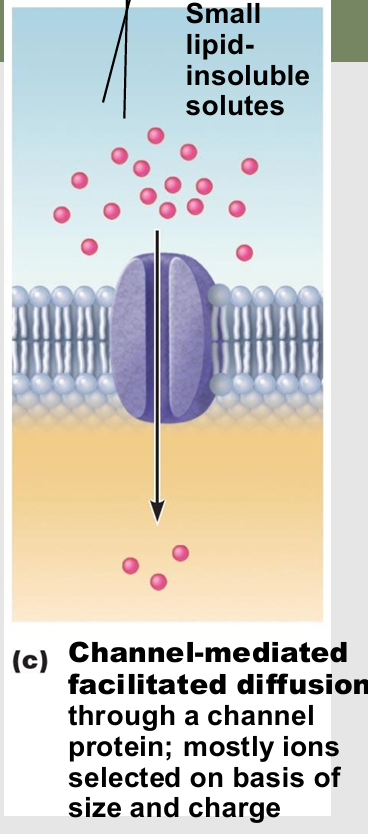
Gated channels
types of channel in Channel-mediated facilitated diffusion
Controlled by chemical or electrical signals
required change
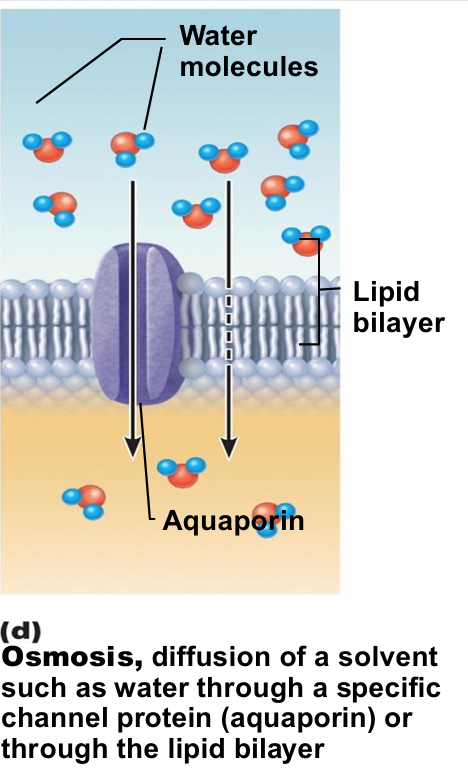
Osmosis
Movement of water into or out of cells down a concentration gradient
passive process
Movement of solvent (e.g., water) across selectively permeable membrane
Water diffuses through plasma membranes
Through lipid bilayer
Through specific water channels called aquaporins (AQPs)
Occurs when water concentration different on the two sides of a membrane
Water concentration varies with number of solute particles because solute particles displace water molecules
Osmolarity
Measure of total concentration of solute particles
Measure of solute concentration
Water moves by osmosis until
hydrostatic pressure and osmotic pressure equalize
hydrostatic pressure
back pressure of water on membrane
osmotic pressure
tendency of water to move into cell by osmosis
When solutions of different osmolarity are separated by membrane permeable to all molecules
both solutes and water cross membrane until equilibrium reached
When solutions of different osmolarity are separated by membrane impermeable to solutes
osmosis occurs until equilibrium reached
Osmosis causes cells to
swell and shrink
Change in cell volume disrupts cell function, especially in neurons
Tonicity
Ability of solution to alter cell's water volume
Types
isotonic
hypertonic
hypotonic
Isotonic
Solution with same non-penetrating solute concentration as cytosol
Hypertonic
Solution with higher non-penetrating solute concentration than cytosol
Hypotonic
Solution with lower non-penetrating solute concentration than cytosol
Sources of intracellular osmolarity:
Macromolecules: Contribute very little to the osmolarity of the cell (few of them compared to small molecules)
Charged, which attracts oppositely charged inorganic ions
Counterions make a major contribution to osmolarity
Small organic molecules (sugars, amino acids, nucleotides):
Both charged small molecules and their counterions contribute to osmolarity
Osmolarity is mainly due to small organic ions
Cell must control osmolarity or water will _
continuously move into the cell by osmosis
Why are Red blood cells a special case of osmolarity
No nucleus
Plasma membrane with a high permeability to water
High number of Na+ - K+ pumps for controlling cell volume
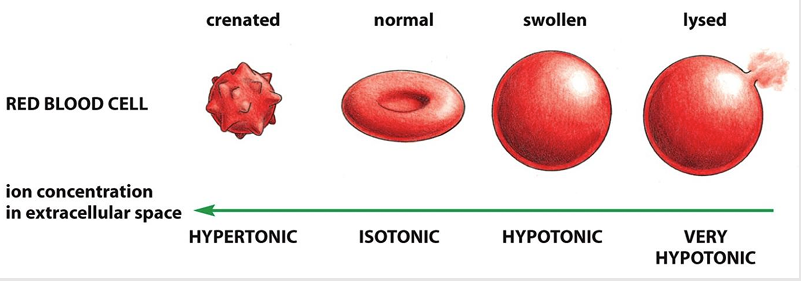
If placed in a hypotonic solution, what will happen to a red blood cell?
Water rushes into cell
Cells burst
(low solute, high water concentration)
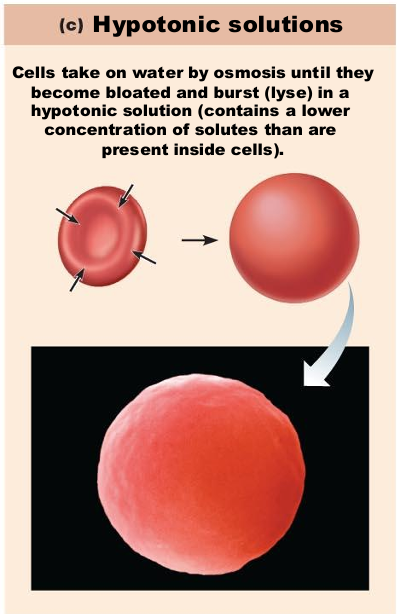
If placed in a hypertonic solution, what will happen to a red blood cell?
Water leaves cell
Cells shrink
(high solute, low water concentration)
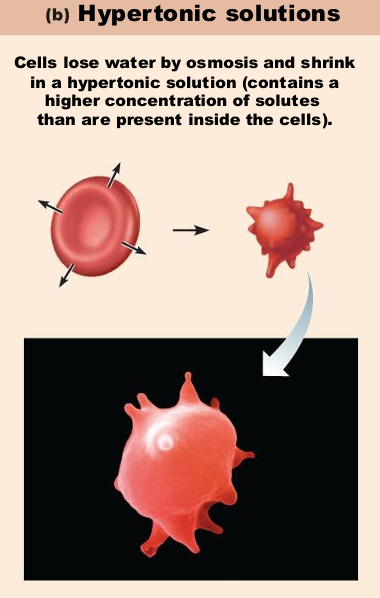
If placed in a isotonic solution, what will happen to a red blood cell?
stay normal
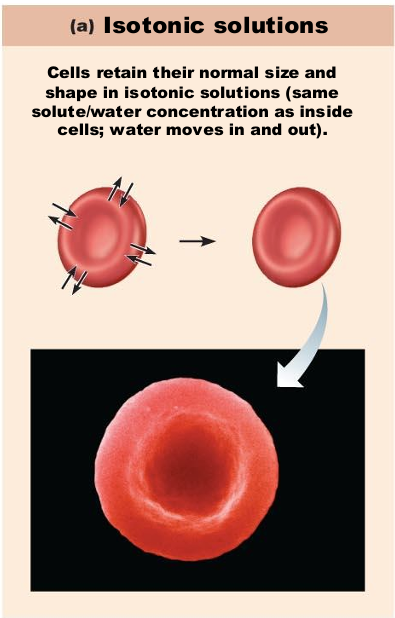
Passive transport allow _
aka: facilitated diffusion
allows solutes to pass the membrane inactively
Uncharged molecule: Transport must be driven by concentration gradient
Charged molecule: Transport drive by concentration gradient and electrical gradient
Most plasma membranes have an electrical potential difference, inside is negative with respect to the outside
Entry of positively charged ions favored
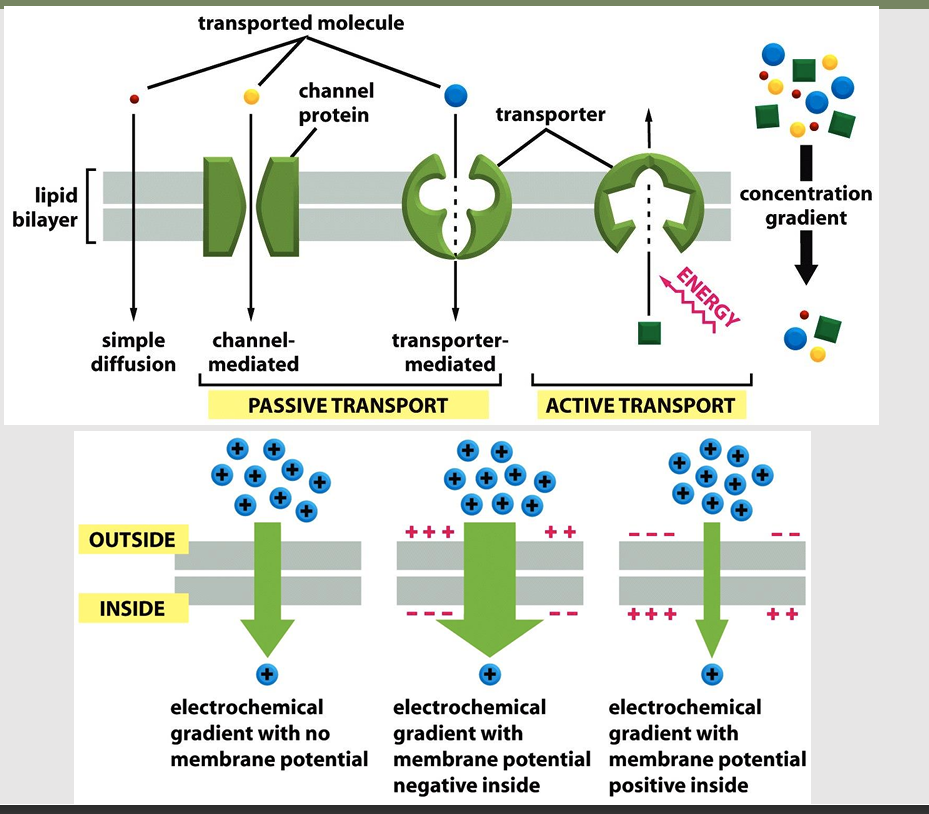
Active transport
Transporter-mediated solute transport against an electrochemical gradient
Requires energy input
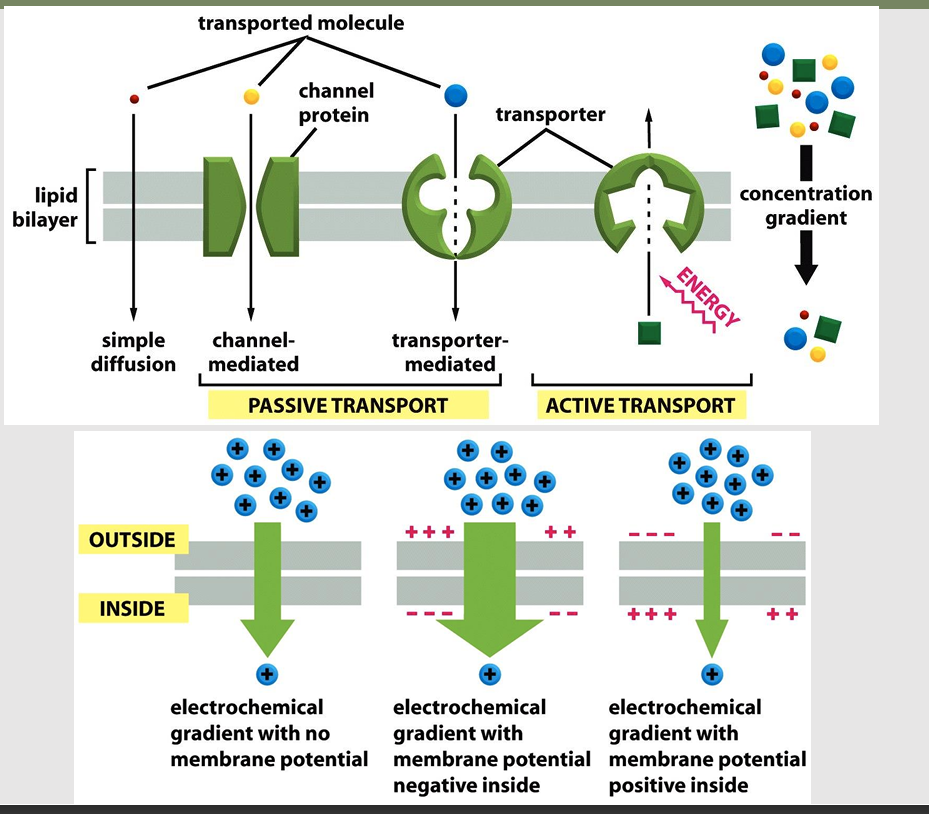
Membrane channels allow _
passive transmembrane movement
Membrane transporters can create _
large differences in intra- and extracellular concentrations
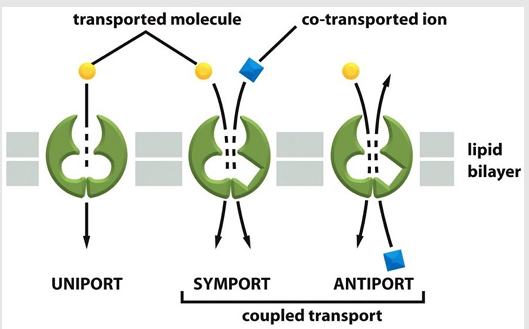
Transporter-mediated solute movement types
Uniporters
Coupled transports
symporters
antiporters
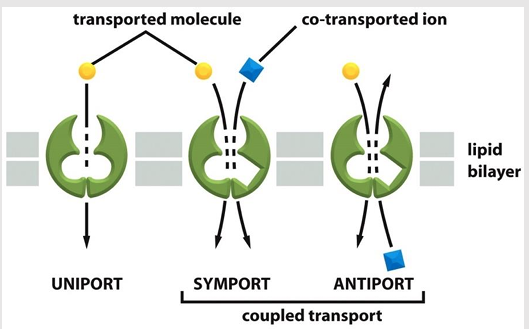
Uniporters
Mediate the movement of a single solute from one side of the membrane to the other at a rate determined by their Vmax and Km
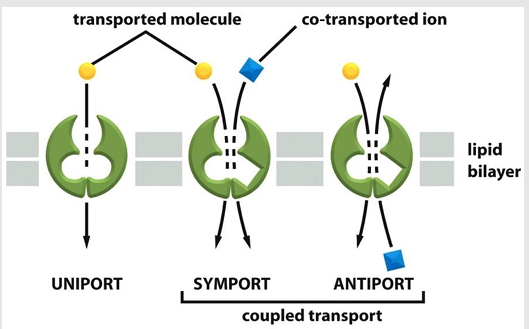
Coupled transporters
Transfer of one solute depends on the transport of a second
Types:
symporters
antiporters
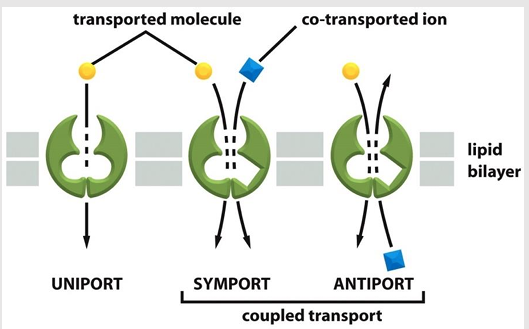
Symporters
type of coupled transporter
aka: co-transporters
Simultaneously transports a second solute in the same direction
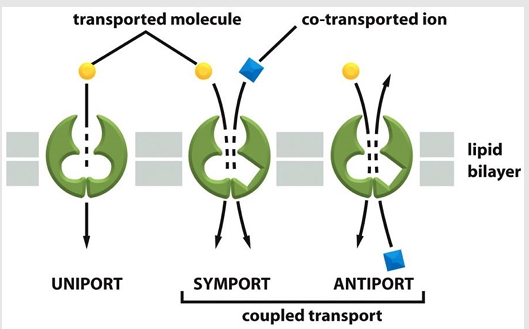
Antiporters
type of coupled transporter
aka: exchangers
Transfers a second solute in the opposite direction
Types of Membrane Transport: Active Processes
Active transport
Vesicular transport
Active Processes require
Both type require ATP to move solutes across a living plasma membrane because
Solute too large for channels
Solute not lipid soluble
Solute not able to move down concentration gradient
Active membrane transport
Process by which a transporter transfers a solute across the lipid bilayer resembles an enzyme-substrate reaction
No modification of solute
Each transporter has one or more binding sites for its solute
Transfers solute by undergoing a conformational change
Vmax of transport = rate at which transporter can flip between its two states
Km = concentration of solute at which transport is half of the maximum rate
Solute binding can be inhibited competitively (other solutes bind) or noncompetitively (inhibitors change the structure of the transporter)
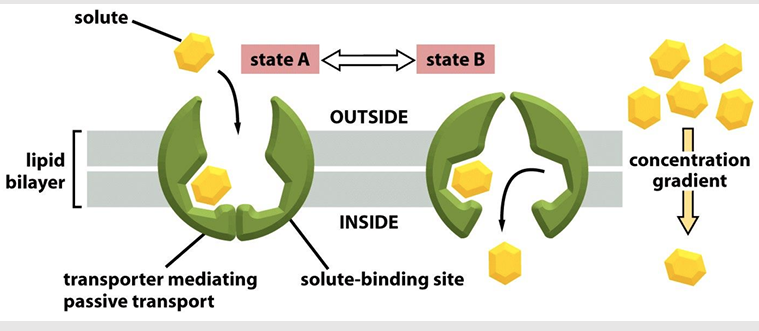
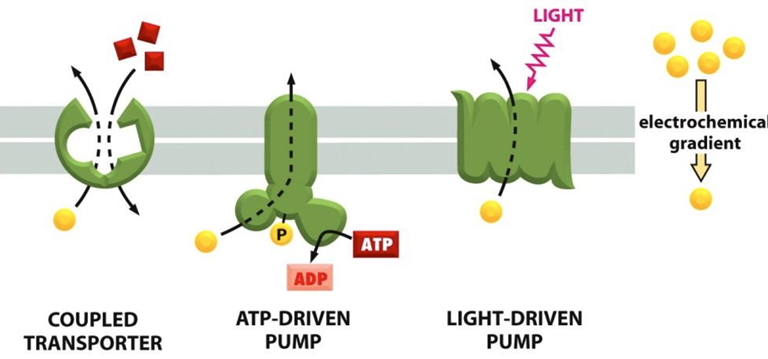
Ways that cells link transporters to an energy source:
Coupled transporters couple the uphill transport of one solute to the downhill transport of another
ATP-driven pumps couple uphill transport to the hydrolysis of ATP
Light driven pumps (bacteria and archaea) couple uphill transport to an input on energy from light
Active transport requires
Requires carrier proteins (solute pumps)
Bind specifically and reversibly with substance
Requires energy
Moves solutes against concentration gradient
Types of active transport:
Primary active transport
Required energy directly from ATP hydrolysis
Secondary active transport
Required energy indirectly from ionic gradients created by primary active transport
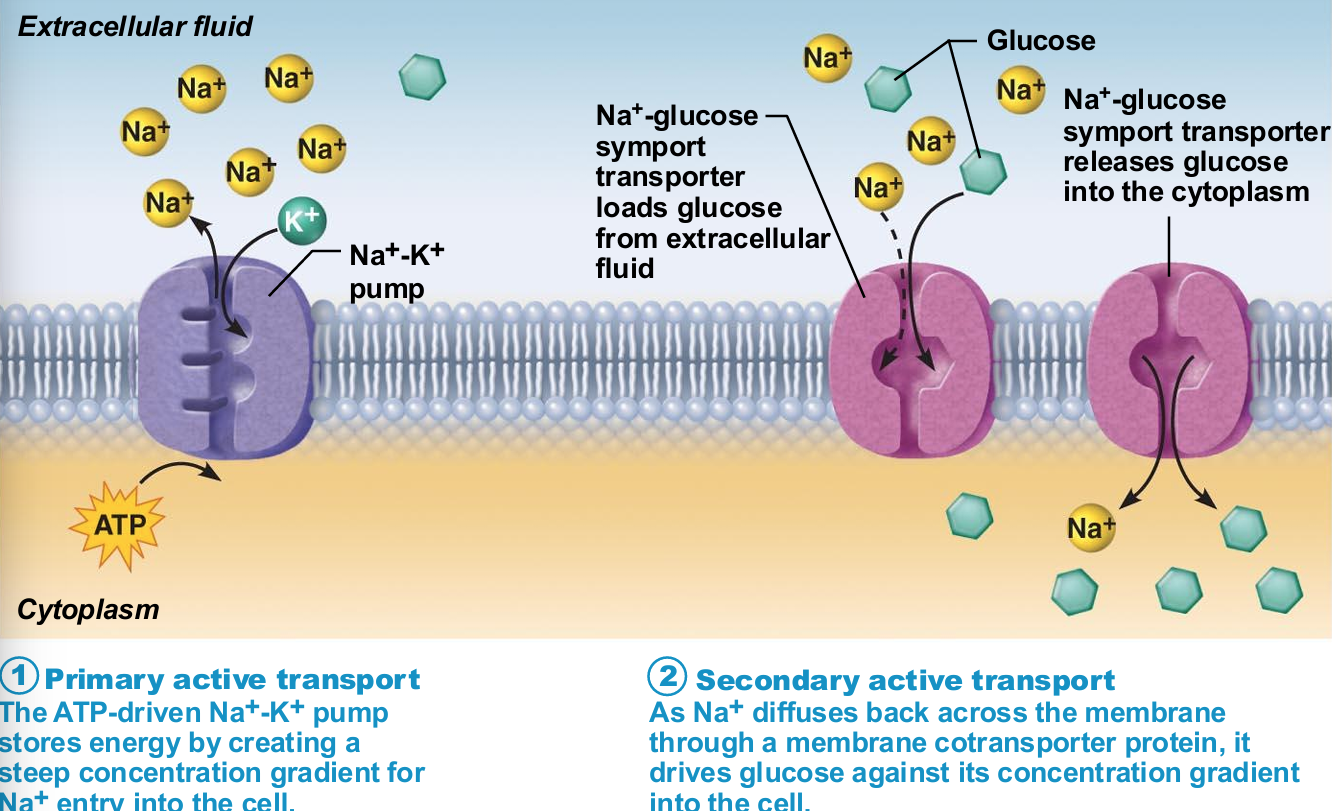
Primary active transport
Energy from hydrolysis of ATP causes shape change in transport protein that "pumps" solutes (ions) across membrane
E.g., calcium, hydrogen, Na+-K+ pumps
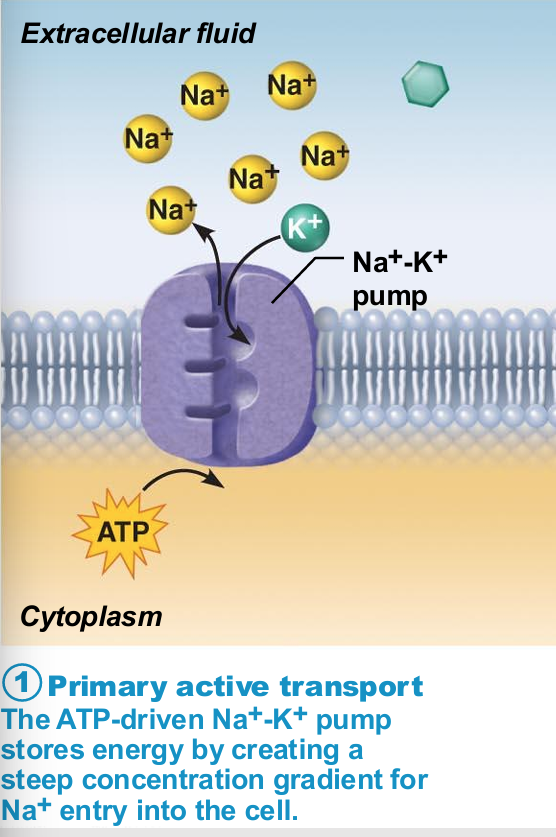
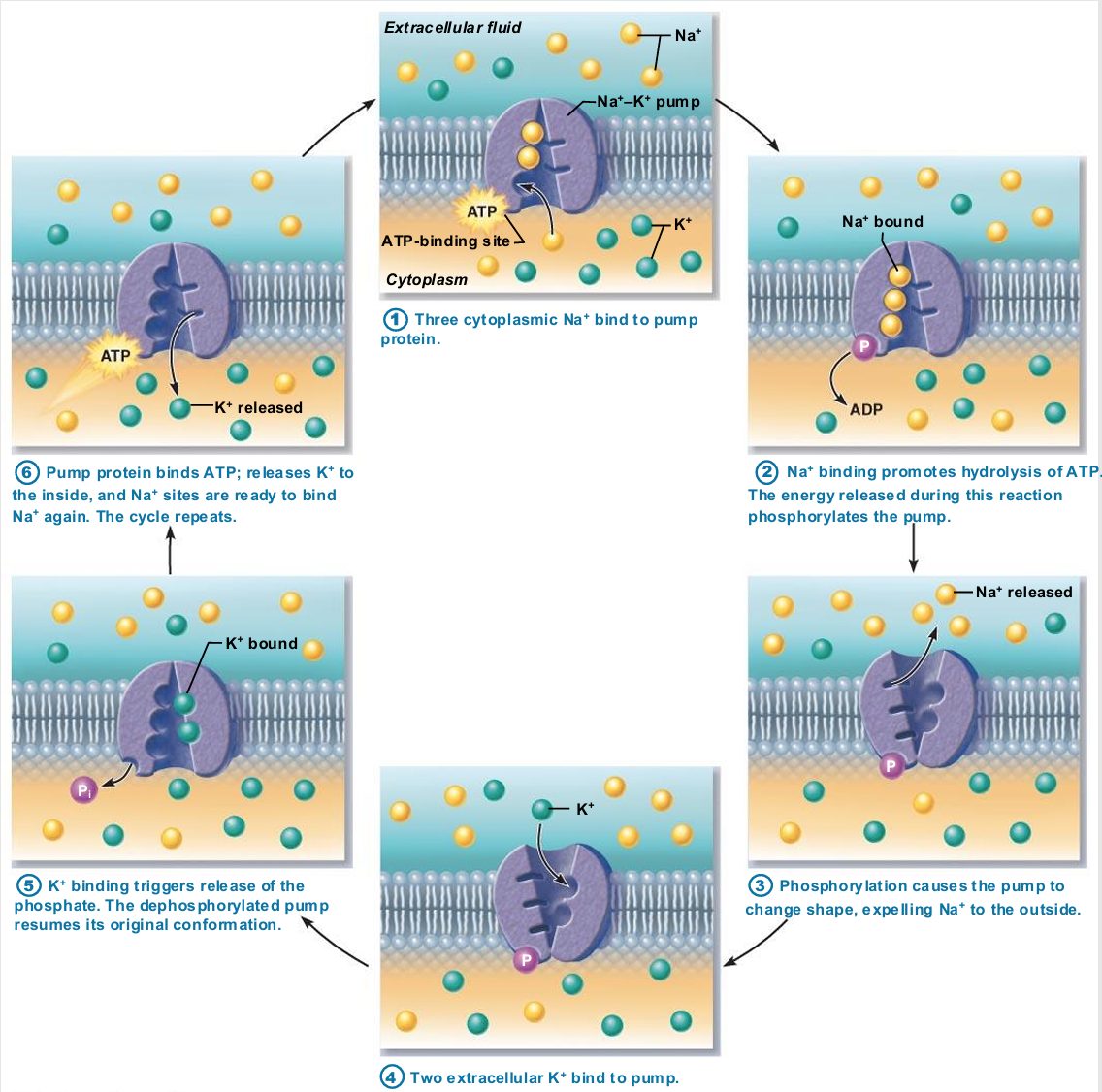
Sodium-potassium pump
Most well-studied
Carrier (pump) called Na+-K+ ATPase
Located in all plasma membranes
Involved in primary and secondary active transport of nutrients and ions
Na+ and K+ channels allow slow leakage down concentration gradients
Na+-K+ pump works as antiporter
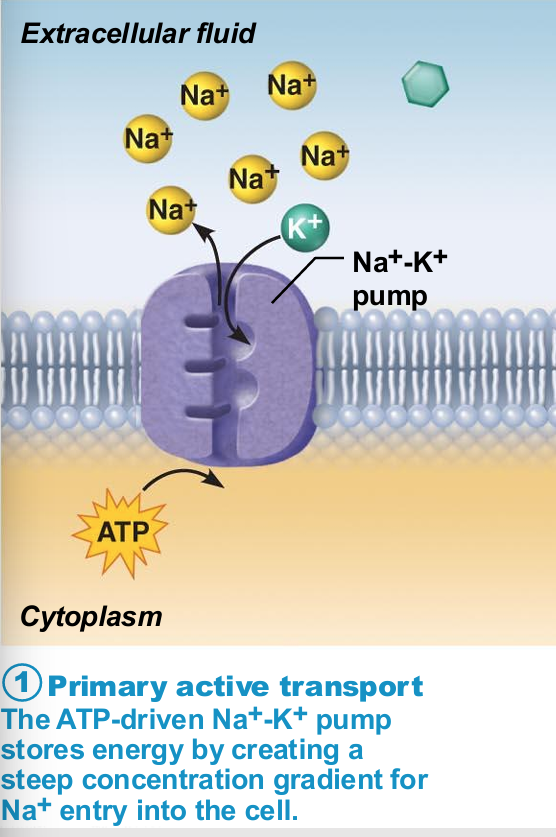
Na+-K+ pump work as antiporter
Pumps against Na+ and K+ gradients to maintain high intracellular K+ concentration and high extracellular Na+ concentration
Maintains electrochemical gradients essential for functions of muscle and nerve tissues
Allows all cells to maintain fluid volume
Generation of a resting membrane potential
Produced by separation of oppositely charged particles (voltage) across membrane in all cells
Cells described as polarized
Voltage (electrical potential energy) only at membrane
Ranges from –50 to –100 mV in different cells
Negative sign indicates inside negative relative to outside
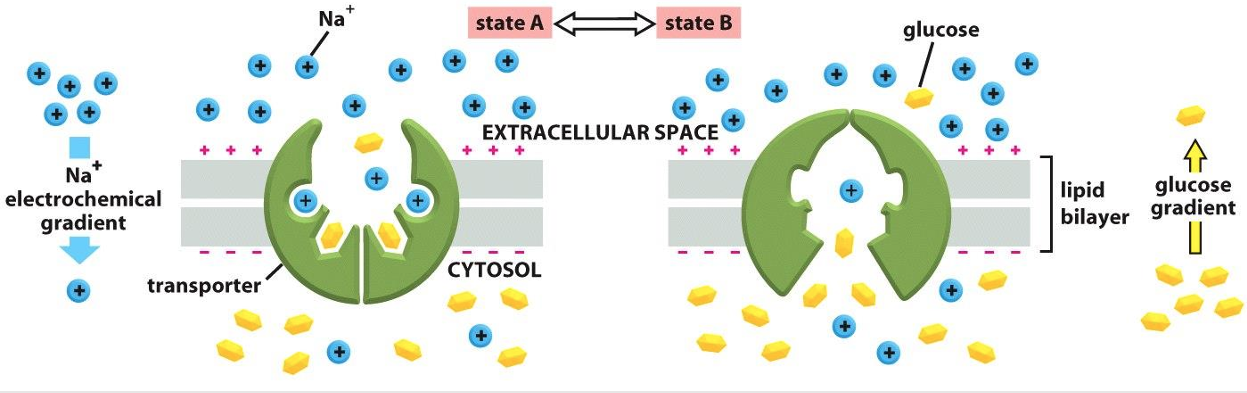
Secondary Active Transport
Depends on ion gradient created by primary active transport
Energy stored in ionic gradients used indirectly to drive transport of other solutes
Cotransport—always transports more than one substance at a time
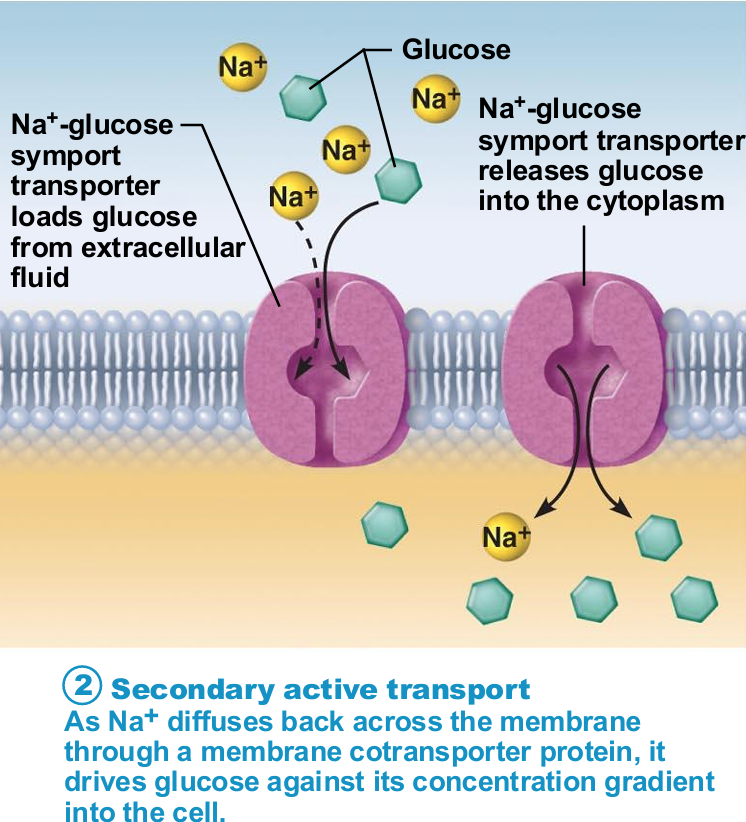
Types of cotransports
Symport system: Substances transported in same direction
Antiport system: Substances transported in opposite directions
Ion gradient active transport
The transfer of two solutes allows the coupled transporters to harvest energy stored in the electrochemical gradient
The free energy released during the movement of an organic ion down an electrochemical gradient is used to pump other solutes uphill against their gradient
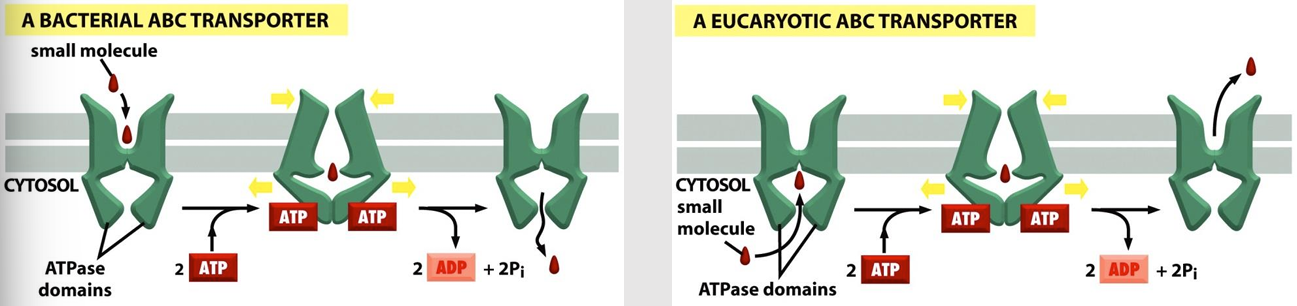
ABC Transporters
(ATP-binding cassettes): Contains two ATP-binding sites
ATP hydrolysis following binding results in conformational changes that alternately expose substrate binding sites to one side of the membrane and then the other
Transport small molecules across the bilayer
Each ABC transporter is thought to be specific for a particular molecule or class of molecules (inorganic ions, amino acids, sugars, peptides, proteins)
ABC transporter clinical importance
Cancer:
Multidrug resistance (MDR) protein:
Able to pump hydrophobic drugs out of the cytosol
Overexpressed in cancer cells
Malaria:
P. falciparum (protist responsible for disease) overexpress the ABC transporter that pumps out chloroquine
Cystic fibrosis:
Caused by a mutation in the gene encoding CFTR
CFTR functions as a Cl- channel in epithelial cells
Irregular ion concentrations in the extracellular fluid
Thick, sticky mucus
Vesicular Transport
Transport of large particles, macromolecules, and fluids across membrane in membranous sacs called vesicles
Requires cellular energy (e.g., ATP)
Vesicular Transport Functions
Exocytosis—transport out of cell
Endocytosis—transport into cell
Phagocytosis, pinocytosis, receptor-mediated endocytosis
Transcytosis—transport into, across, and then out of cell
Vesicular trafficking—transport from one area or organelle in cell to another
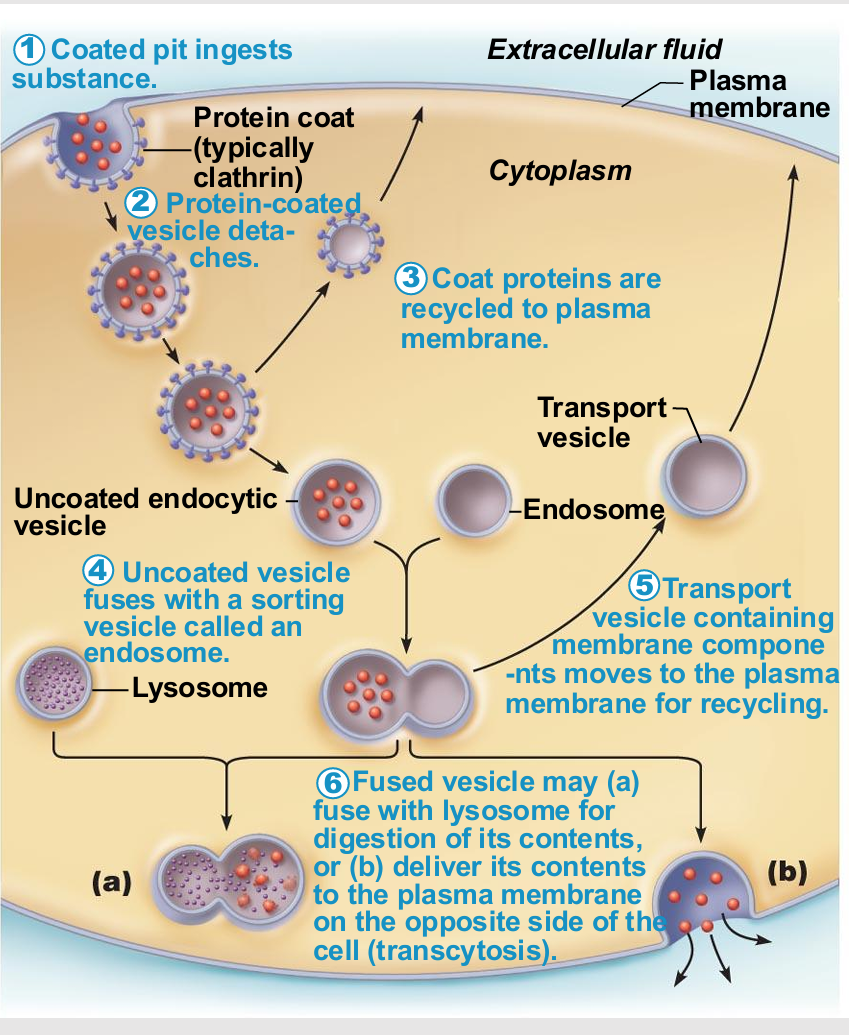
Endocytosis and transcytosis similarities
Involve formation of protein-coated vesicles
Often receptor mediated, therefore very selective
Some pathogens also hijack for transport into cell
Once vesicle is inside cell it may
Fuse with lysosome
Undergo transcytosis
Endocytosis Types
Phagocytosis (cell eating)
Pinocytosis (cell drinking)
Receptor-mediated endocytosis
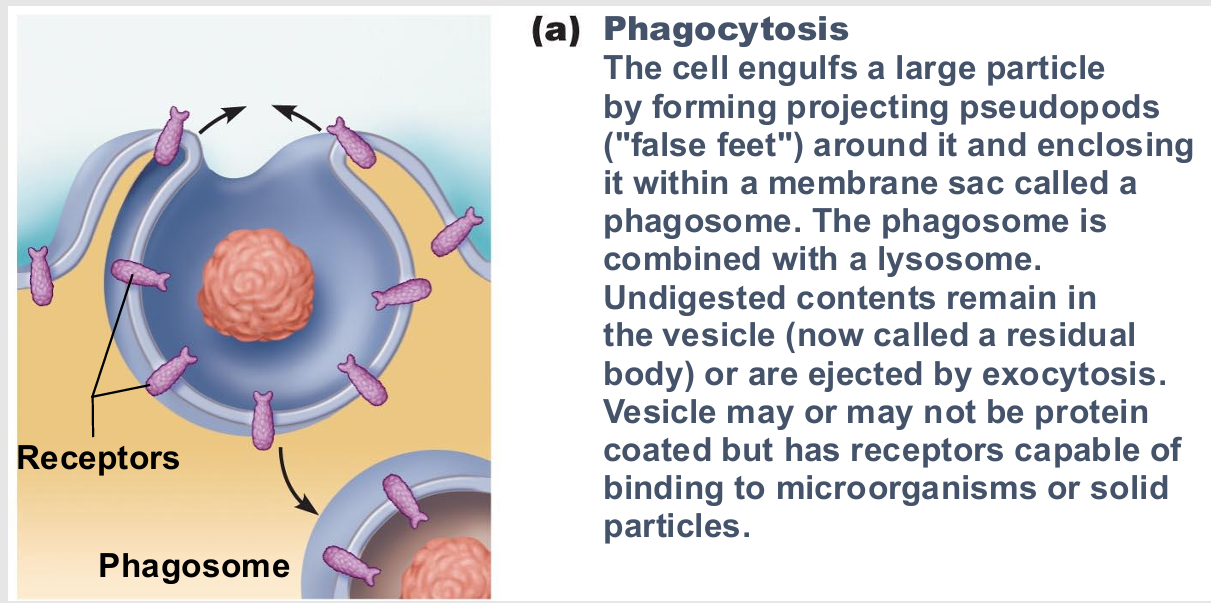
Phagocytosis
type of endocytosis
cell eating
Pseudopods engulf solids and bring them into cell's interior
Form vesicle called phagosome
Used by macrophages and some white blood cells
Move by amoeboid motion
Cytoplasm flows into temporary extensions
Allows creeping
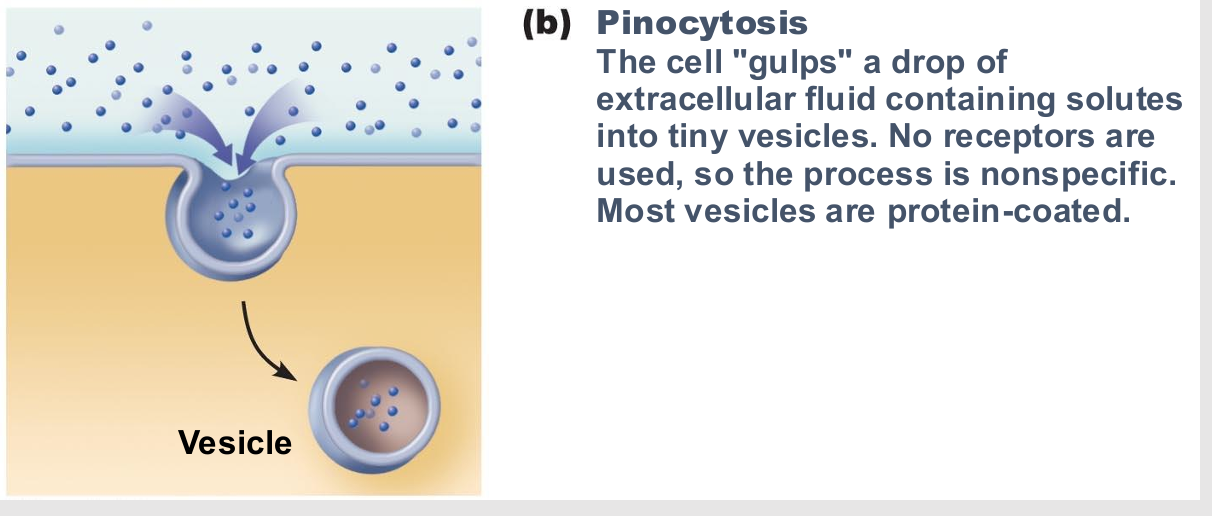
Pinocytosis
fluid-phase endocytosis
cell drinking
Plasma membrane infolds, bringing extracellular fluid and dissolved solutes inside cell
Fuses with endosome
Most cells utilize to "sample" environment
Nutrient absorption in the small intestine
Membrane components recycled back to membrane
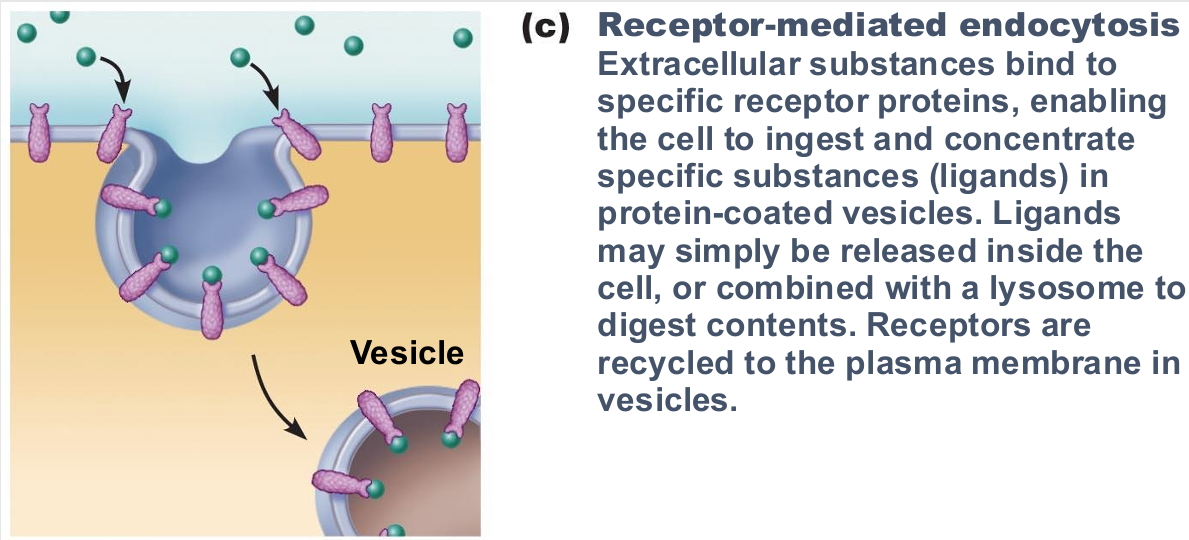
Receptor-mediated endocytosis
Allows specific endocytosis and transcytosis
Cells use to concentrate materials in limited supply
Clathrin-coated pits provide main route for endocytosis and transcytosis
Uptake of enzymes, low-density lipoproteins, iron, insulin, and, unfortunately, viruses, diphtheria, and cholera toxins
Different coat proteins
Caveolae
Coatomer
Caveolae
coat protein involved in receptor-mediated endocytosis
Capture specific molecules (folic acid, tetanus toxin) and use transcytosis
Involved in cell signaling but exact function unknown
Coatomer
coat protein involved in receptor-mediated endocytosis
Function in vesicular trafficking
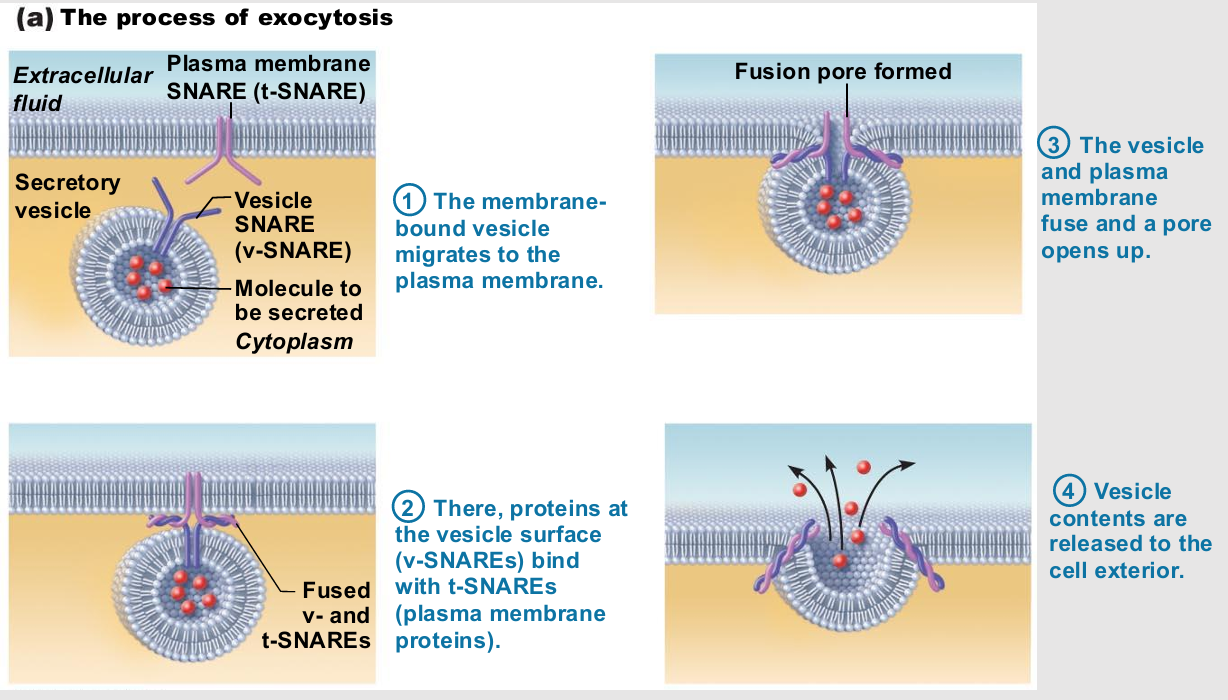
Exocytosis
Very targeted
Cell barding
Usually activated by cell-surface signal or change in membrane voltage
Substance enclosed in secretory vesicle
v-SNAREs ("v" = vesicle) on vesicle find
t-SNAREs ("t" = target) on membrane and bind
Functions
Hormone secretion, neurotransmitter release, mucus secretion, ejection of wastes
glycocalyx
"Sugar coating" at cell surface
Lipids and proteins with attached carbohydrates (sugar groups)
Every cell type has different pattern of sugars
Specific biological markers for cell to cell recognition
Allows immune system to recognize "self" and "non self"
Cell binding/communities
Some cells "free"
e.g., blood cells, sperm cells
Some bound into communities
Cell junction types
ways cells are bound
Tight junctions
Desmosomes
Gap junctions
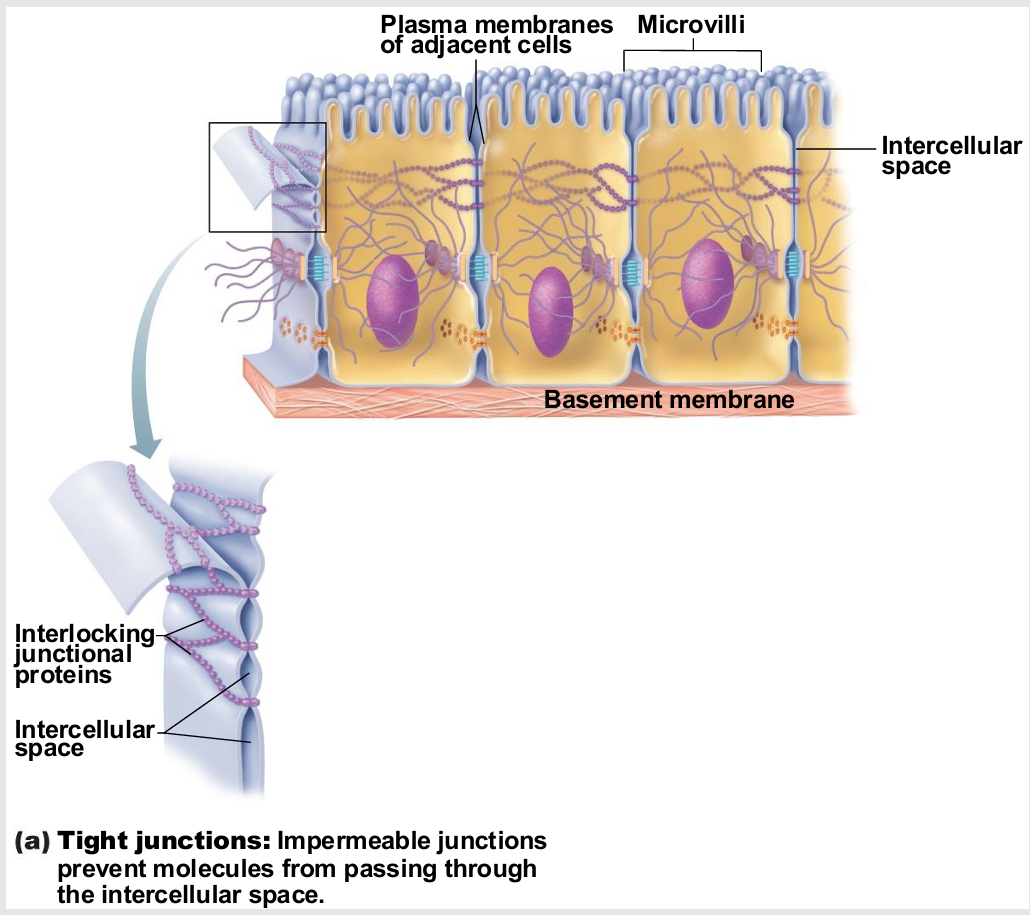
Tight Junctions
Adjacent integral proteins fuse → form impermeable junction encircling cell
Prevent fluids and most molecules from moving between cells
chemical barrier
Where might these be useful in body?
digestive system
placenta (fine control)
blood vessels
epithelial
endothelial
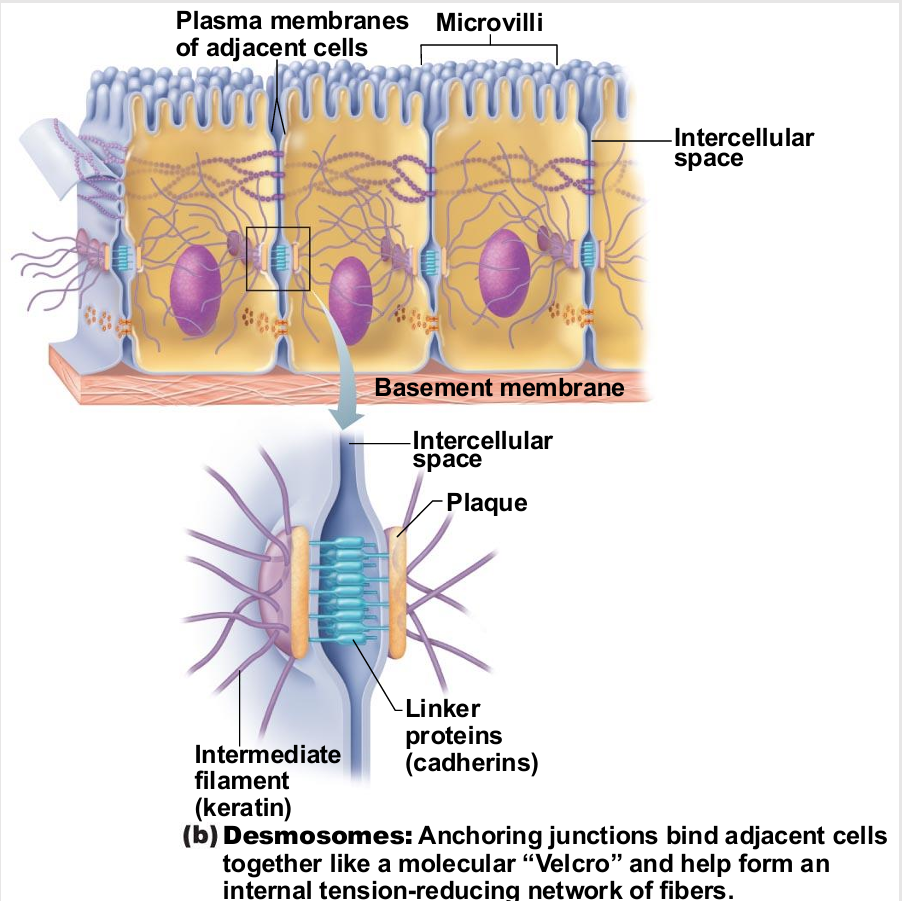
Desmosomes
"Rivets" or "spot-welds" that anchor cells together at plaques (thickenings on plasma
membrane)
Linker proteins between cells connect plaques
Keratin filaments extend through cytosol to opposite plaque giving stability to cell
Reduces possibility of tearing (mechanical)
Where might these be useful in body?
muscles
skin
ligaments
tendons
tension re-enforcement
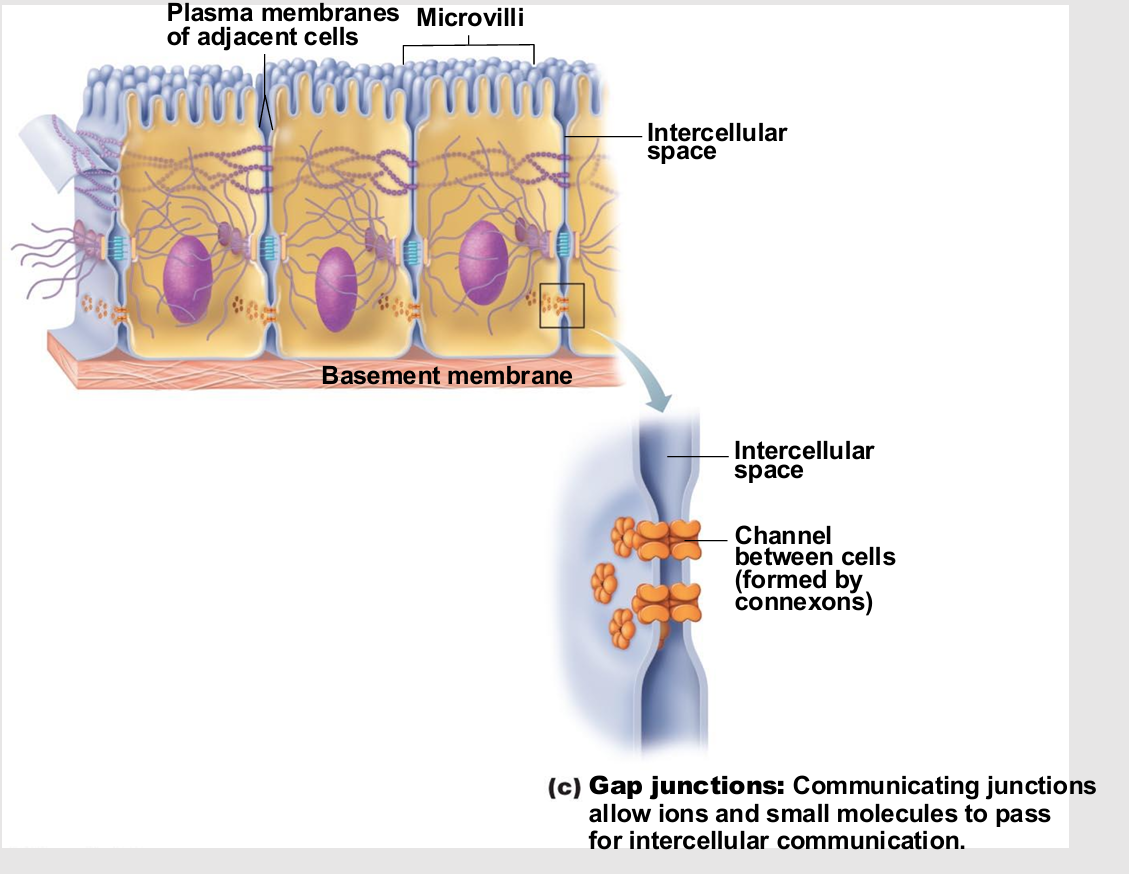
Gap Junctions
Transmembrane proteins form pores (connexons) that allow small molecules to pass from cell to cell
For spread of ions, simple sugars, and other small molecules between cardiac or smooth muscle cells
Where might these be useful in body?
cardiac
muscle cells
Cell Environment Interactions
Cells interact directly or indirectly by responding to extracellular signals
Always involves glycocalyx
Cell adhesion molecules (CAMs)
Plasma membrane receptors
Voltage-gated channel proteins
Roles of Cell Adhesion Molecules
Thousands on approximately every cell in body
Anchor to extracellular matrix or each other
Assist in movement of cells past one another
Attract WBCs to injured or infected areas
Stimulate synthesis or degradation of adhesive membrane junctions
Transmit intracellular signals to direct cell migration, proliferation, and specialization
Roles of Plasma Membrane Receptors
contact signaling
chemical signaling
Contact signaling (Plasma Membrane Receptors)
touching and recognition of cells
e.g., in normal development and immunity
Chemical signaling (Plasma Membrane Receptors)
interaction between receptors and ligands (neurotransmitters, hormones, and paracrines) to alter activity of cell proteins (e.g., enzymes or chemically gated ion channels)
Same ligand can cause different cell responses
Response determined by what receptor linked to inside cell
Chemical signaling (Plasma Membrane Receptors) Process
Catalytic receptor proteins become activated enzymes
Chemically gated channel-linked receptors open and close ion gates → changes in excitability
G protein–linked receptors activate G protein, affecting an ion channel or enzyme, or causing release of internal second messenger, such as cyclic AMP
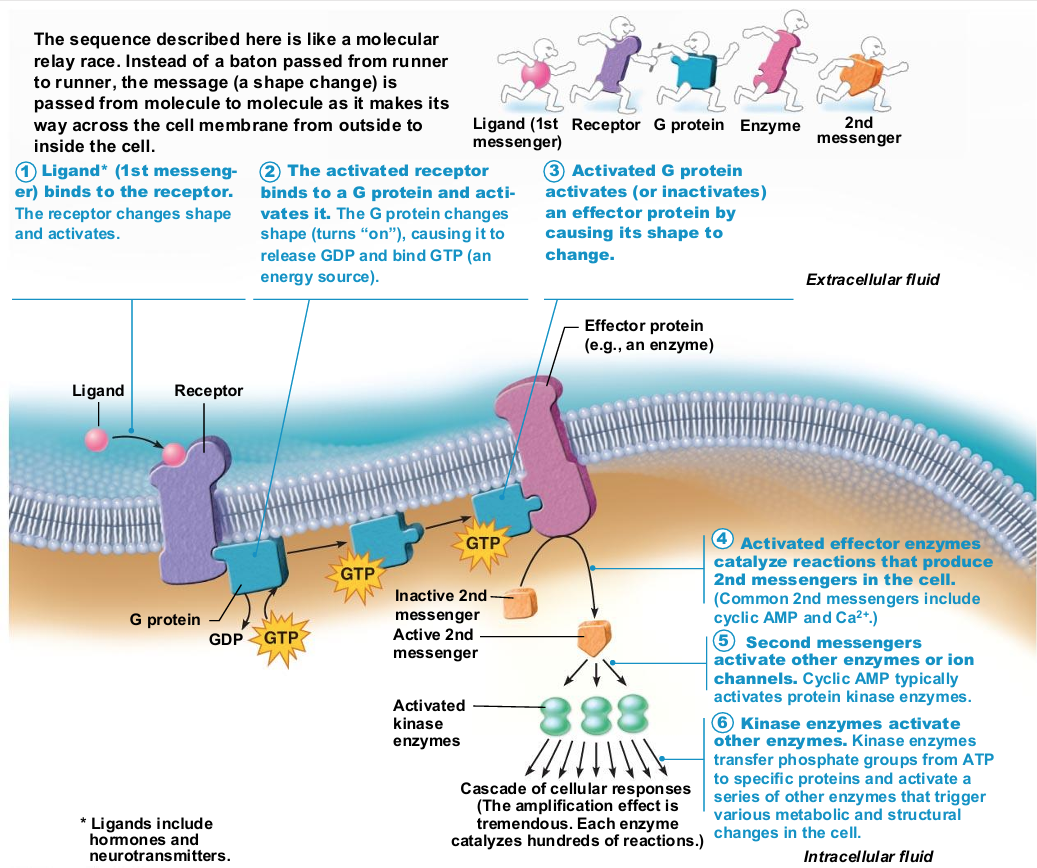
Integral proteins
Firmly inserted into membrane (most are transmembrane)
Have hydrophobic and hydrophilic regions
Can interact with lipid tails and water
Function as transport proteins (channels and carriers), enzymes, or receptors
Function of integral proteins
Transport
Signal Transduction
Attachment
Enzymatic activity
Cell-cell junctions
Recognition
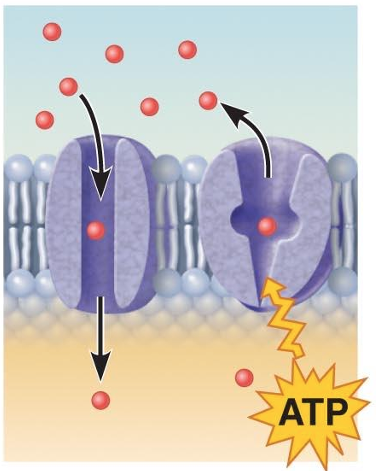
Transport (integral proteins)
A protein (left) that spans the membrane may provide a hydrophilic channel across the membrane that is selective for a particular solute.
Some transport proteins (right) hydrolyze ATP as an energy source to actively pump substances across the membrane
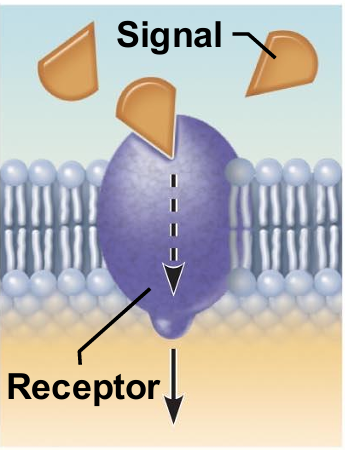
integral proteins as receptors for signal transduction
A membrane protein exposed to the outside of the cell may have a binding site that fits the shape of a specific chemical messenger, such as a hormone.
When bound, the chemical messenger may cause a change in shape in the protein that initiates a chain of chemical reactions in the cell.
Attachment (integral proteins)
Attachment to the cytoskeleton and extracellular matrix
Elements of the cytoskeleton (cell's internal supports) and the extracellular matrix (fibers and other substances outside the cell) may anchor to membrane proteins, which helps maintain cell shape and fix the location of certain membrane proteins.
Others play a role in cell movement or bind adjacent cells together.
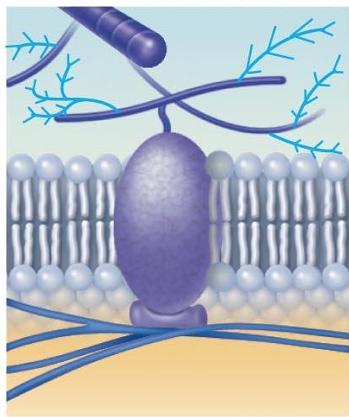
Intercellular joining (integral proteins)
Membrane proteins of adjacent cells may be hooked together in various kinds of intercellular junctions.
Some membrane proteins (cell adhesion molecules or CAMs) of this group provide temporary binding sites that guide cell migration and other cell-to-cell interactions.
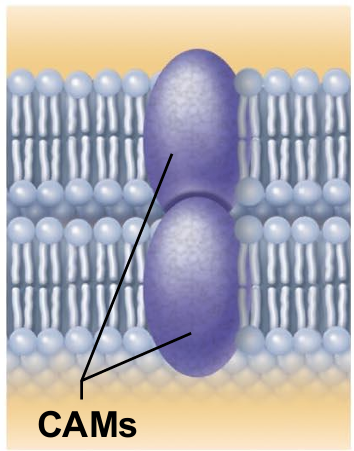
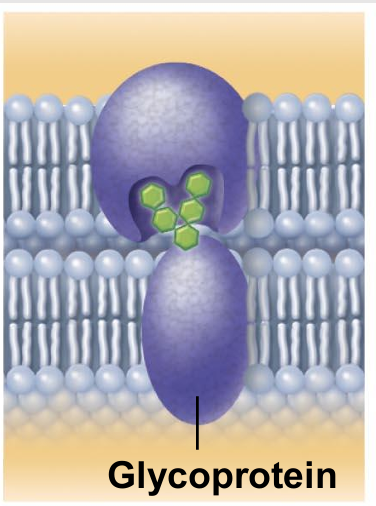
Cell-cell recognition (integral proteins)
Some glycoproteins (proteins bonded to short chains of sugars) serve as identification tags that are specifically recognized by other cells.
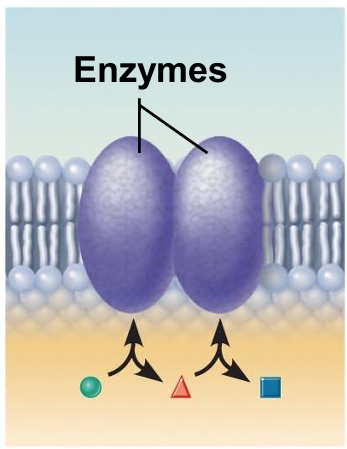
Enzymatic activity (integral proteins)
A membrane protein may be an enzyme with its active site exposed to substances in the adjacent solution.
A team of several enzymes in a membrane may catalyze sequential steps of a metabolic pathway as indicated (left to right) here.