Biology: Atomic Structure, Chemical Bonds, and Microscopy Techniques
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
Chemistry
The study of interactions between atoms and molecules.
Atom
The smallest unit of matter that cannot be subdivided into smaller substances without losing its properties.
Molecule
A structure formed when atoms interact to combine.
Compound
A molecule that contains two or more kinds of atoms.
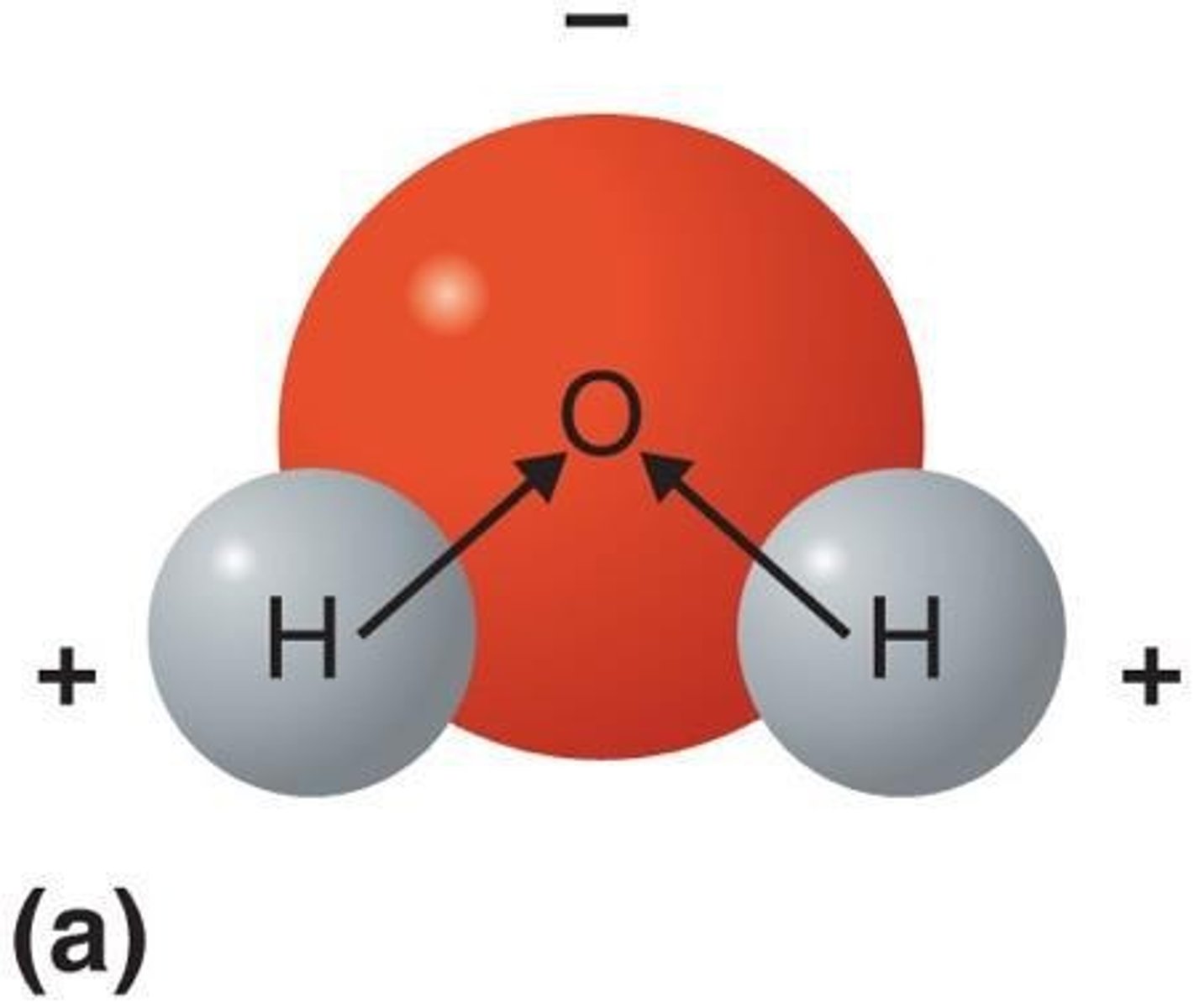
Water
A compound consisting of two atoms of hydrogen and one atom of oxygen (H2O).
Atomic number
The number of protons in the nucleus of an atom.
Atomic mass
The total number of protons and neutrons in an atom.
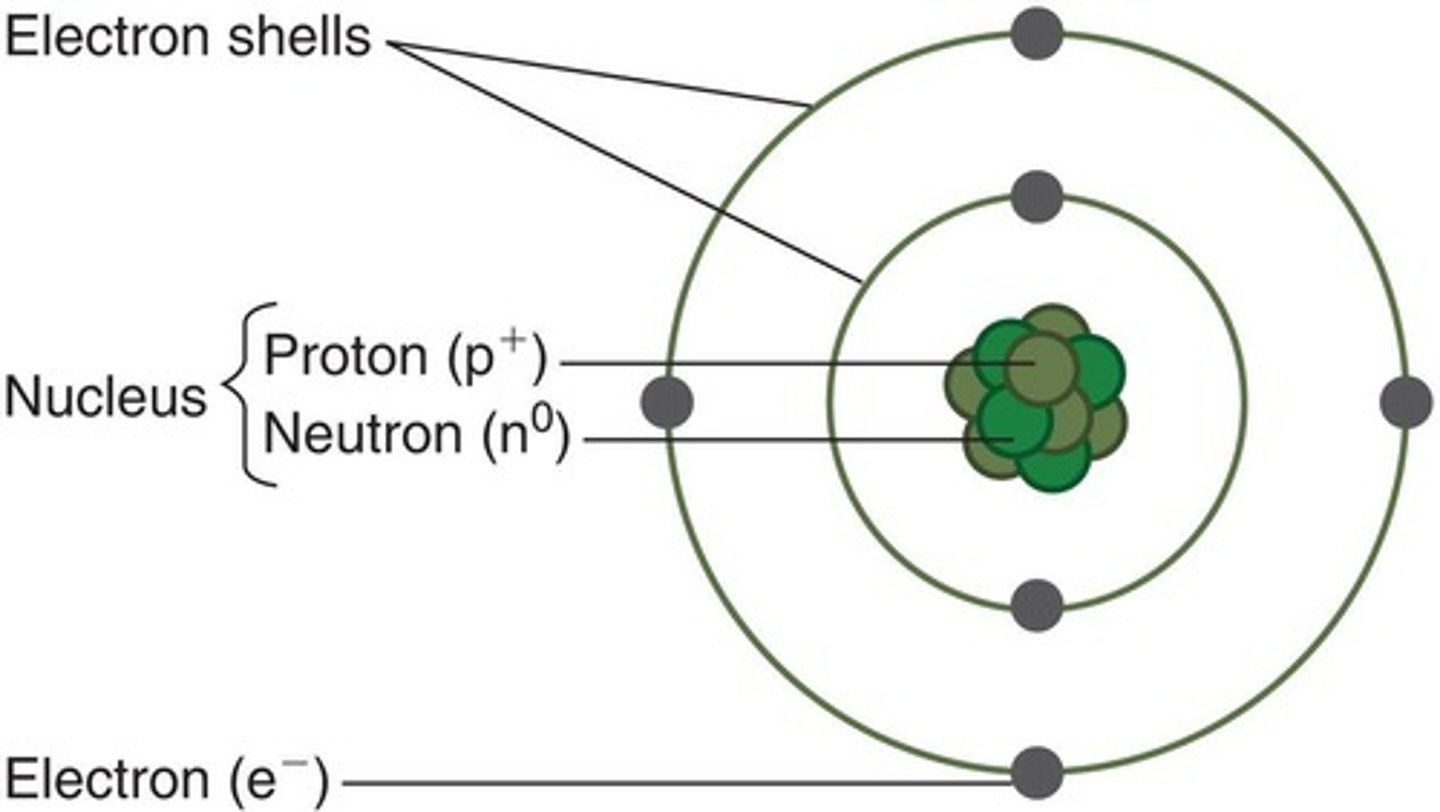
Electron shells
Arrangements of electrons corresponding to different energy levels.
Electron configuration
The arrangement of electrons in electron shells.
Innermost shell
The electron shell that can hold up to 2 electrons.
Second shell
The electron shell that can hold up to 8 electrons.
Third shell
The electron shell that can hold up to 8 electrons if it is the outermost shell.
Valence shell
The outermost shell of an atom that determines its reactivity with other atoms.
Valence
The number of missing or extra electrons in the outermost shell, representing the combining capacity of an atom.
Chemical bonds
Attractive forces formed by the valence electrons of combining atoms.
Hydrogen atom structure
An H atom contains a nucleus and one electron shell with one electron and one unfilled space.
Carbon atom structure
A C atom contains a nucleus and two electron shells, with 2 electrons in the innermost shell and 4 electrons in the outermost shell.
Nitrogen atom structure
An N atom contains a nucleus and two electron shells, with 2 electrons in the innermost shell.
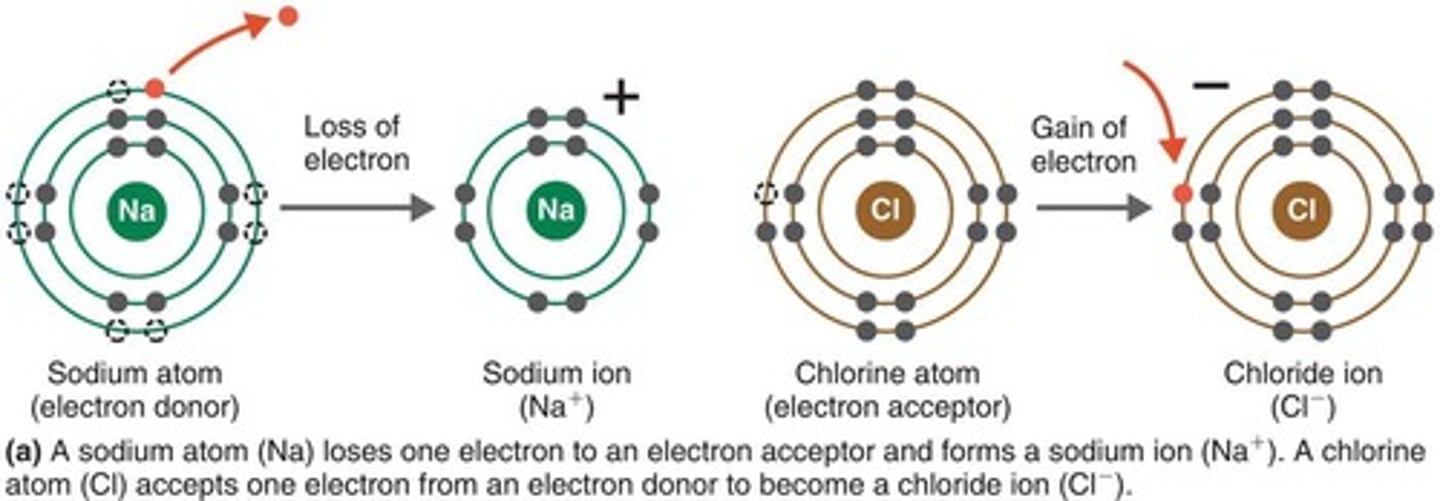
Ionic Bond
An attraction between ions of opposite charge that holds them together to form a stable molecule. Weaker ionic bonds are important in biochemical reactions such as antigen-antibody reactions.
Covalent Bond
A bond formed by two atoms that share one or more pairs of electrons. Covalent bonds are stronger and more common in organisms than ionic bonds. They are very common and are responsible for holding together the atoms of most molecules in organisms.
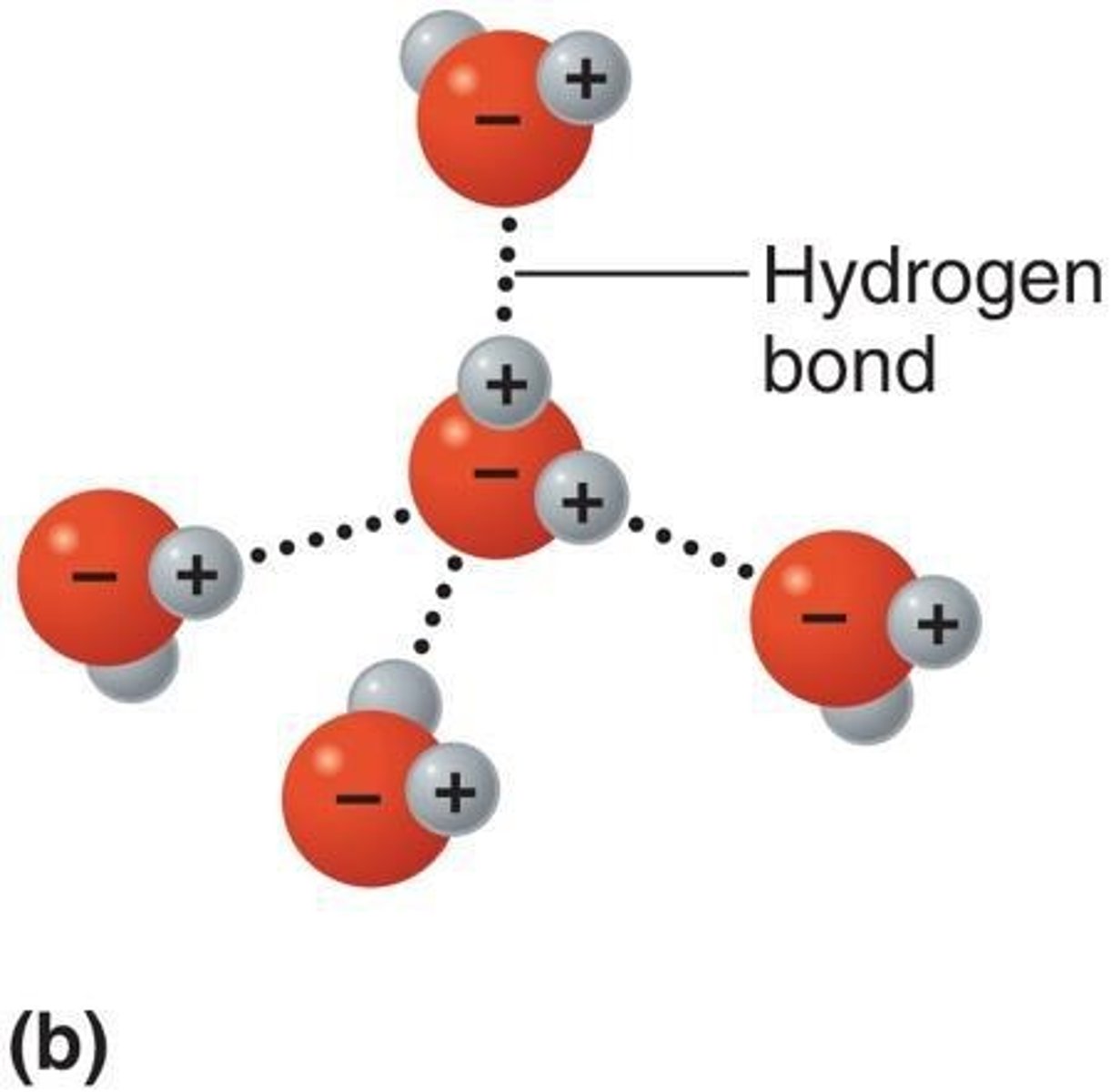
Hydrogen Bond
A relatively weak bond in which a hydrogen atom that is covalently bonded to one oxygen or nitrogen atom is attracted to another oxygen or nitrogen atom. Hydrogen bonds do not bind atoms into molecules, but serve as bridges between different molecules or different portions of the same molecule, for example, within proteins and nucleic acids, to stabilize the structures.
Chemical Reaction
A process in which one or more substances (reactants) are transformed into one or more different substances (products) through the breaking and forming of chemical bonds.
Endergonic Reaction
A reaction that absorbs more energy than it releases.
Exergonic Reaction
A reaction that releases more energy than it absorbs.
Anabolism
The synthesis of molecules in a cell.
Catabolism
Includes the decomposition reactions in a cell.
Hydrogen Bonding in Water
A water molecule can form hydrogen bonds with up to four nearby water molecules.
Water Characteristics
Attributed to its polarity and hydrogen-bonding, including being an excellent temperature buffer and solvent.
Solutes in Solutions
Polar and ionic substances undergo dissociation in water, forming solutes in solutions.
Surface Tension
Created by the hydrogen bonding between water molecules.
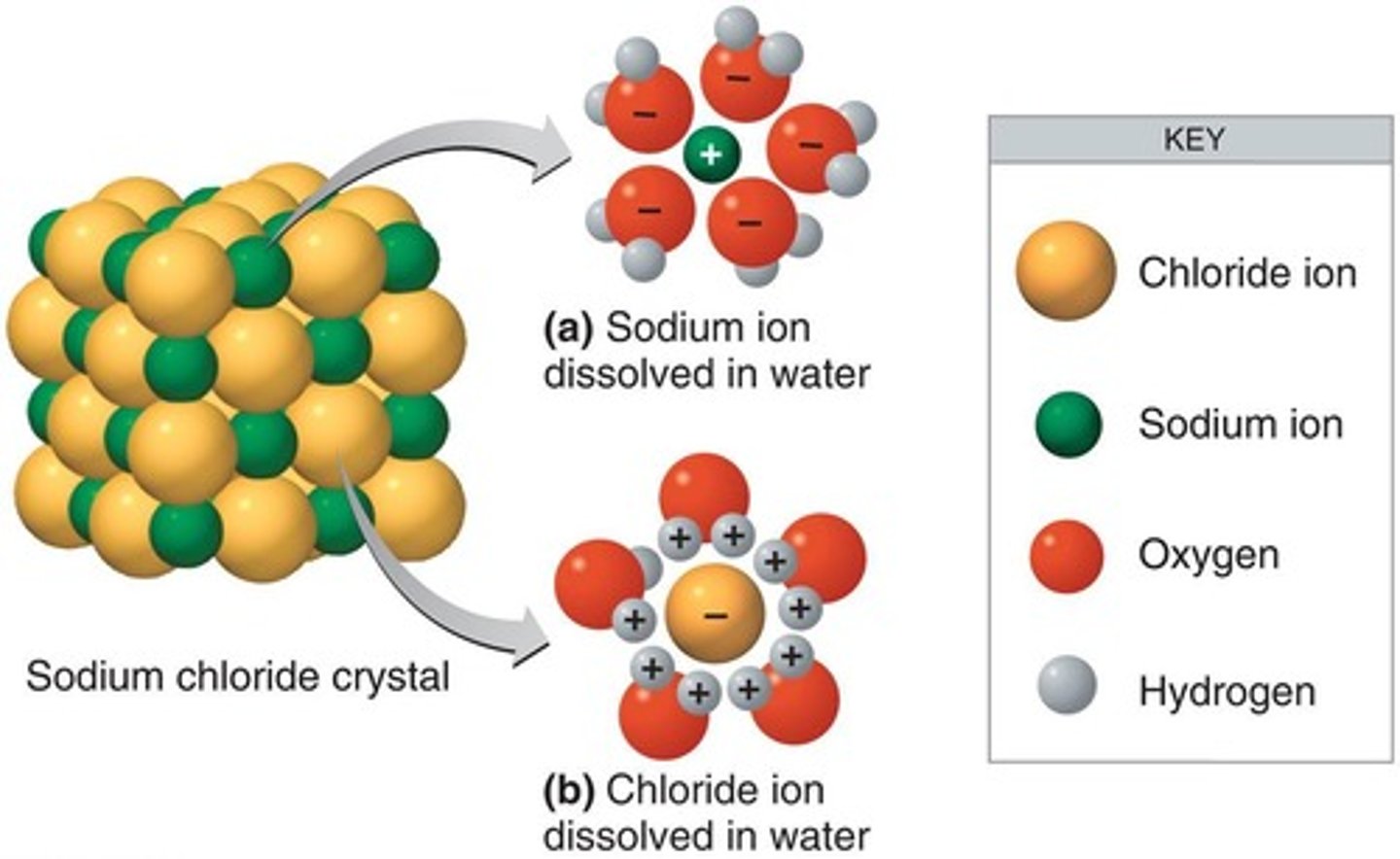
Sodium Chloride in Water
Describes how water acts as a solvent for sodium chloride (NaCl).
Oxygen Atom Structure
The O atom contains a nucleus and two electron shells, with the nucleus depicted as a large filled red circle labeled O.
Outer Electron Shell
The outermost shell has two single electrons and two pairs of electrons depicted as filled black circles.
Carbohydrates
Cellular energy sources and components of cell structures.
Lipids
Nonpolar molecules that are insoluble in water, serving as primary structural components of cell membranes and energy storage.
Proteins
Polymers of amino acid building blocks that are essential for cell structure and function.
Nucleic Acids
Molecules that carry genetic information, including DNA and RNA.
Monosaccharides
The simplest form of carbohydrates, consisting of single sugar molecules.
Disaccharides
Carbohydrates formed from two monosaccharides.
Polysaccharides
Carbohydrates that are made up of long chains of monosaccharide units.
Fats
Lipids that contain glycerol and fatty acids.
Phospholipids
Lipids composed of glycerol, two fatty acids, and a phosphate group; they are amphipathic.
Steroids
A type of lipid characterized by a carbon skeleton with four fused rings; cholesterol is an example.
Amino Acids
Building blocks of proteins, with 20 different types available.
Denaturation
The process that leads to the loss of shapes and functions of proteins.
Enzymes
Proteins that speed up chemical reactions.
Transport Proteins
Proteins that move chemicals across membranes.
Receptor Proteins
Proteins embedded in the cell membrane that bind to specific external or internal signals.
Flagella
Structures that aid in the movement of cells.
Exotoxins
Some bacterial toxins that can be proteins.
Cytoskeleton-like Elements
Structural components of cells that provide support and shape.
Nucleotides
The building blocks of nucleic acids, consisting of a five-carbon sugar, a phosphate group, and a nitrogen-containing base.
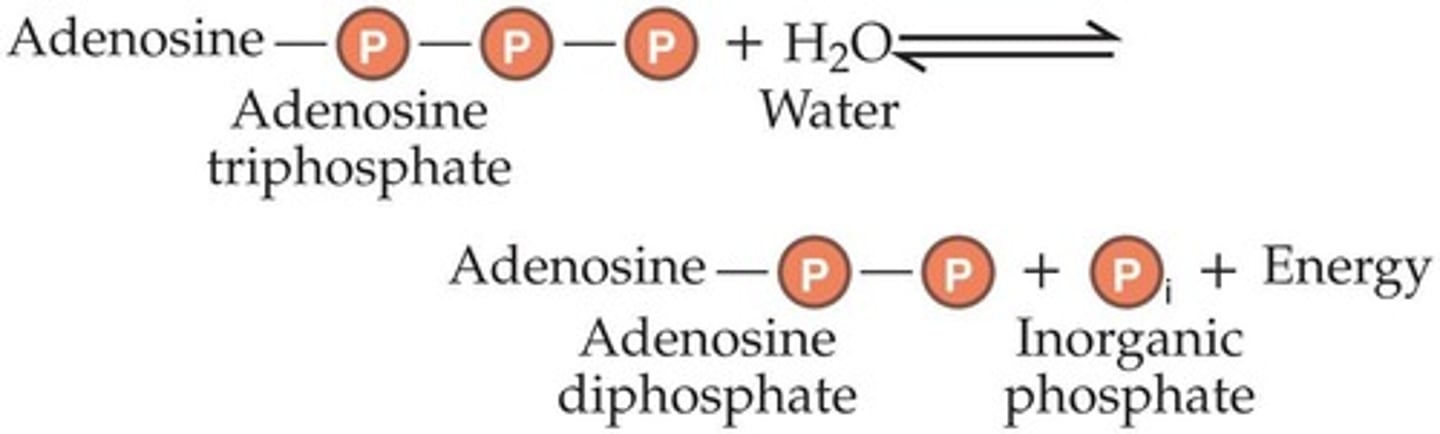
Adenosine Triphosphate (ATP)
The principal energy-carrying molecule of all cells, storing energy in high-energy bonds.
Micrometers
Units of measurement for microorganisms, where 1 μm = 10^-6 m.
Nanometers
Units of measurement for microorganisms, where 1 nm = 10^-9 m.
Total Magnification
The product of the objective lens and ocular lens in a microscope.
Resolution
The ability of lenses to distinguish two points or fine detail and structure.
Limit of Resolution
The smallest distance at which two points can be distinguished, which is 0.2 μm (200 nm) for a compound light microscope.
Resolving Power
Calculated as λ/2NA, where λ is the wavelength and NA is the numerical aperture of the lens.
Refractive index
Measure of the light-bending ability of a medium.
Immersion oil
Used to keep light from refracting.
Fluorescence Microscopy
Uses UV (short wavelength) light source of illumination.
Fluorescent substances
Absorb UV light and emit longer wavelength (visible) light.
Fluorescent dyes
Used to stain cells that do not naturally fluoresce.
Fluorochrome
A fluorescent dye that can stain cells bright yellow, green, or orange against a dark field of view.
Fluorescent-antibody (FA) technique
Also known as immunofluorescence; uses antibodies specific for a type of microbial pathogen tagged with a fluorochrome.
Pathogenic microbe detection
Fluorescent antibodies adhere to the microbe, causing it to fluoresce when viewed with fluorescence microscopy.
Electron Microscopy
Uses electrons instead of light for imaging.
Transmission Electron Microscopy
A beam of electrons passes through ultrathin sections of a specimen.
Scanning Electron Microscopy
An electron gun produces a beam of electrons that scans the surface of an entire specimen.
Magnification of Transmission Electron Microscopy
10,000-10,000,000x.
Limit of resolution for Transmission Electron Microscopy
0.2 nm.
Magnification of Scanning Electron Microscopy
1000-500,000x.
Limit of resolution for Scanning Electron Microscopy
0.5 nm.
Simple stain
Highlights the entire microorganism to visualize cell shapes and structures.
Differential Stains
Used to distinguish between bacteria.
Special Stains
Used to distinguish parts of microorganisms.
Gram stain
Classifies bacteria into Gram-positive or Gram-negative.
Acid-fast stain
Used for the identification of Mycobacterium and Nocardia.
Examples of Special Stains
Capsule stain, Endospore stain, Flagella stain.