RAM studying
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
Z
Atomic number
Ion
charged atom
N
neutrons
Different N and Z
Isotope
A
Atomic mass, N+Z
Chart of nuclides horizontal
Chart of nuclides vertial
N
Z
Chart of nuclides: gray and white
Gray is stable, white artificial, black line natural
CHart of nuclides diagram
Nuclei with a mass number, A, less than 20 tend to be stable when N = Z; nuclei with mass numbers greater than 200 tend to be stable when N = 1.5 × Z;
Decay in relation to line of stability
, those to the right of the line will undergo β − decay to reduce their neutronproton ratios. Likewise, nuclei to the left will use β + decay or electron capture to increase their neutron-proton ratios. Nuclides that are just too heavy to be stable will emit alpha particles to reduce their weight
Alpha characteristic
Kicking out an alpha particle carries away a certain amount of binding energy, so a particular nuclide will emit alpha particles of characteristic energy
Beta minus:
Neutron turns to proton, e-, and antineutrino
When does electron capture occur?
What is it?
When nucleus doesn’t have enough E to perform beta decay
Inner electron into nucleus, releases x-ray.
Gamma:
ray emitted when shifting energy states of nucleus
Isonomer
add m for notation, different nucleus arrangements of a compound
some excited states have measurable lifetimes; atoms in these states are said to be isomers or metastable states of the ground state atom
Helf life units
1/sec
Activity equation:
A=(half life)N if N=nuclei in sample
A = A0e −λt
Ionization and excitation
Ionization occurs when a charged particle passes an atom closely enough for its electric field to remove an orbital electron and create an ion pair. Excitation occurs when the charged particle excites an orbital electron into a higher energy level, and the excited electron releases its energy by emitting an X-ray when it returns to its normal energy level.
Phtoelectric effect
Photoelectric: Photon hit electron making e- higher E level. Sometimes it frees from atom entirely. This is the photoelectric effect, when a sufficiently energetic photon transfers all its energy into an orbiting electron. A free electron, typically referred to as a photoelectron, and a positive ion are left behind.
Kinetic energy of photoelectric effect
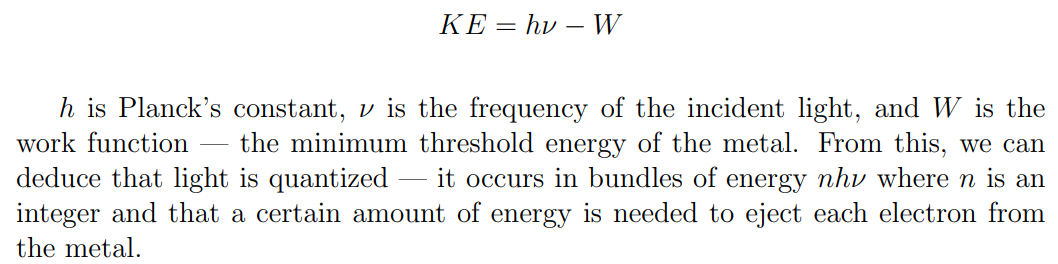
Compton scattering:
Exothermic vs endothermic
exo creates E, endo consumes E
Sheilding:
Under most circumstances, only gamma shielding and neutron shielding are considered because alpha and beta particles have comparatively small ranges in most material. In special circumstances, extra precautions are taken against beta radiation.
Beta shielding
Most beta particles are stopped by a 1 cm thickness of unit density material, e.g., water, plastic, or tissue. Common beta shields are wood or plastic. There are dangers due to bremstrahlung and backscattering
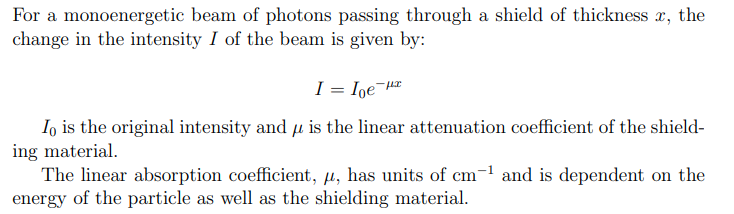
Gamma and x-ray shielding equation
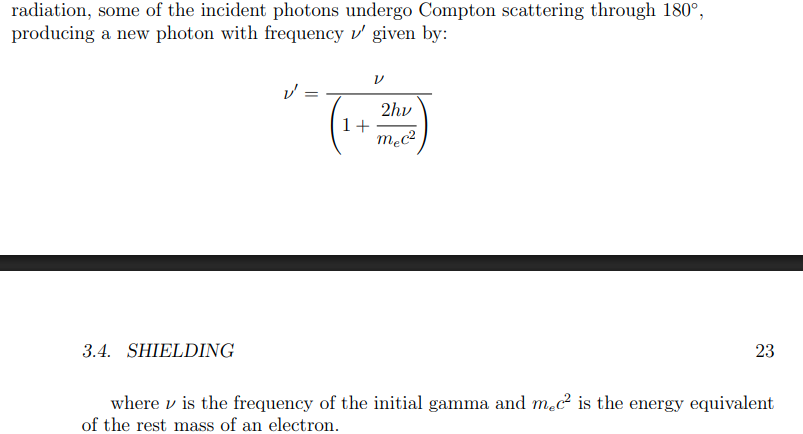
Backscattering equation
Buildup
Compton scattering and pair production increasing radiation on the far side of a shield
Tritium (3H)
half life = 12 years. Beta minus. Ingestion. Urinalysis, wipe test, liquid scintillation.
Bio half life 10 days
Scintillation drawbacks:
cannot detect small E bata or alpha unless liquid scintellation
PIC
leaf. Instantaneous reading, inaccurate (20%).
EPD
Affected by electromagnetic radiation from high voltage equipment or 2 way radios. 10% innacuracy
TLD (thermal)
wide response range, reusable.
OPtically stimulated luminesence (OSL)
sam proccess as TLD. Wider range, permanent reading.
Carbon 14
Half life = 5730 years. Beta minus. ingestion, skin absorbtion. urinalysis, breath measurements, gm tube, wipe test w/liquid scintillation.
Bio half life = 40 days
Phosphorous 32
(p-32)
Half life: 14.3 days. Beta minus. ingestion, skin absorbtion. Film, dosimeter rings, urinalysis, GM tube.
Dose rate @ 10 cm = 2.7 rad/hr. No led.
Use extra caution.
Sulfur-35
half life 87 days. Beta minus. ingestion, skin absorbtion. Urinalysis, gm tube, liquid scint counter. VOlitile.
I-125
Half life: 60 days. Electron capture, gamma. Ingestion, skin absorbtion. Film badge, dosimeter rings, wipe test. Use lead shielding. 2 pairs of gloves.