PS231: Neurophysiology IV
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Voltage gated sodium channel
Crucial for generating action potentials
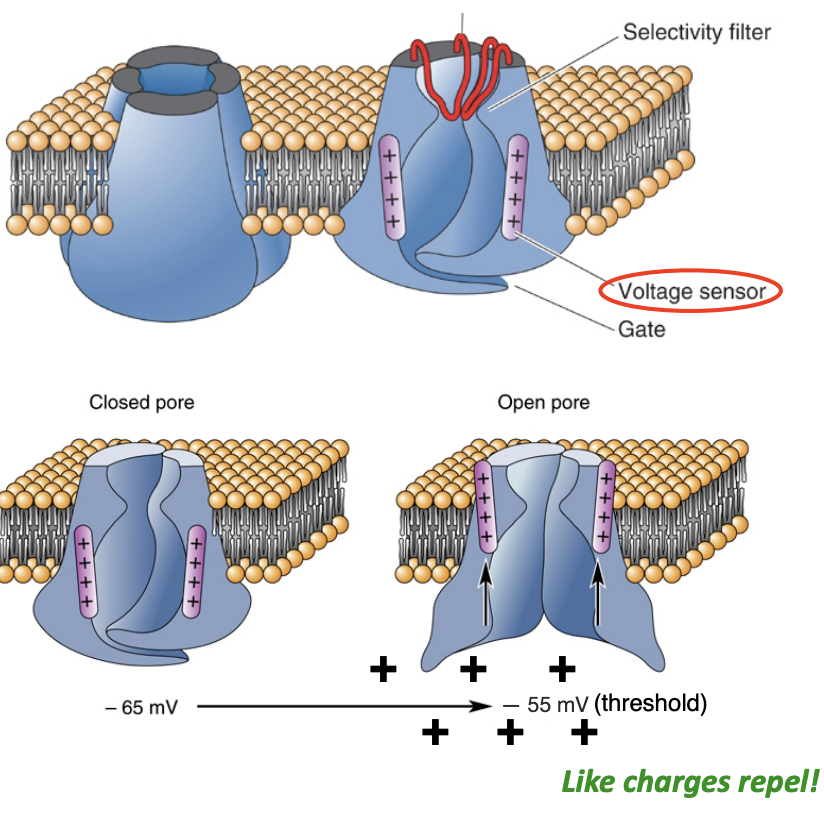
Voltage sensor [Voltage-gated sodium channel (VGNa+)]
Has positively charged residues in S4 region (“voltage sensor”)
This allows the protein channel to twist open when a depolarization invades the axon hillock (like charges repel)
This twisting results in an opening of the pore that only allows the passage of sodium ions (selectivity filter)
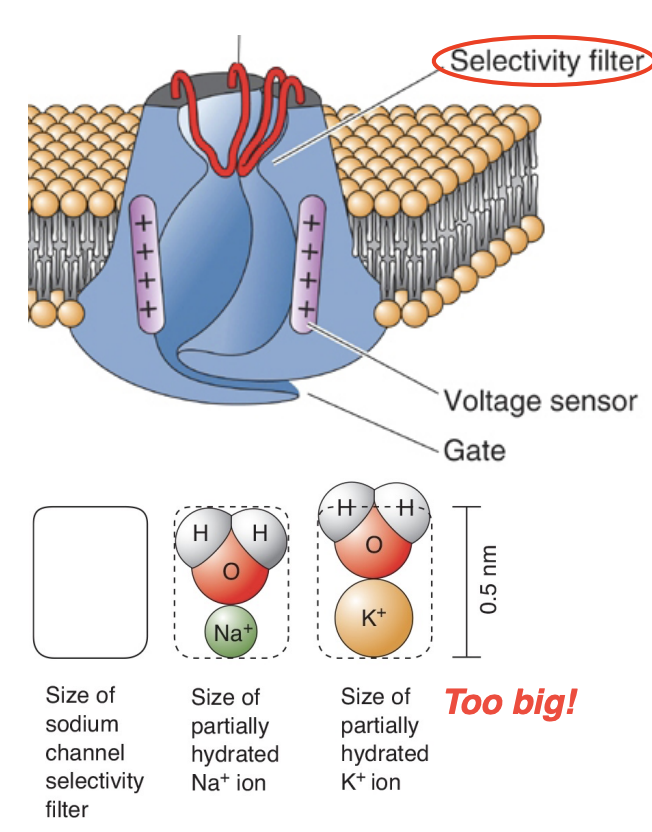
Selectivity for sodium ions [Voltage-gated sodium channel (VGNa+)]
The VGNa+ channel is ~12x more permeable to Na+ than K+ (pore
loop selectivity filter)
Selectivity for Na+ ions is based on size, charge, and energy of hydration of the ion (basically, the hydrated K+ ion doesn’t fit through the pore)
Functional Properties:
They open FAST
They only stay open for ~1 ms before they inactivate
They cannot be opened again by depolarization until the membrane voltage resets (absolute refractory period)
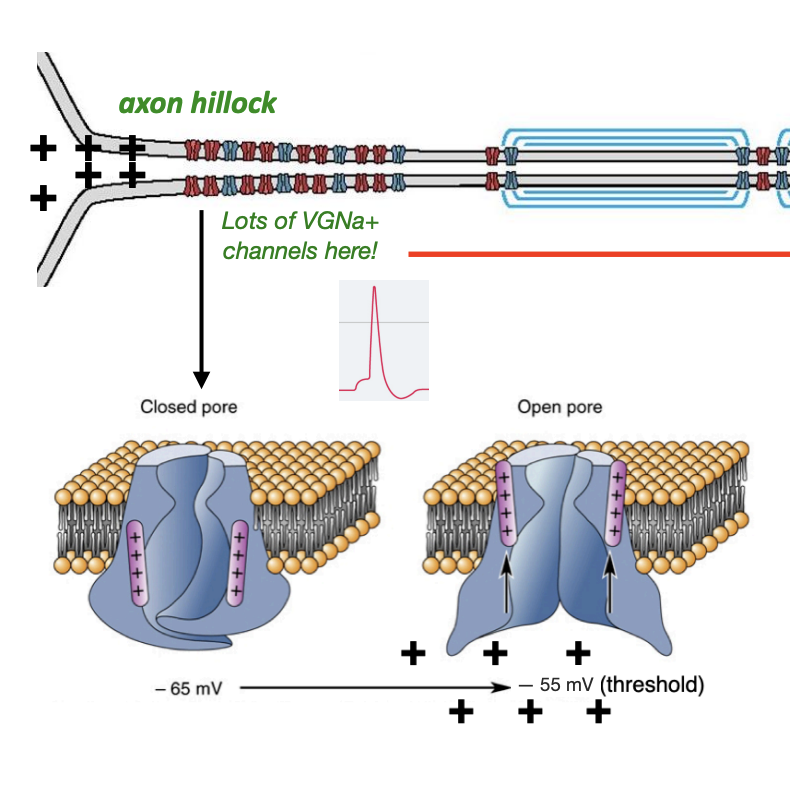
VGNa+ Channels Start the Action Potential Off at the Axon Hillock
As positive charge starts to build up in the dendrites/cell body as a result of excitation from excitatory presynaptic inputs, the membrane becomes more and more depolarized (more positively charged)
At around -55 mV, the neuron reaches “threshold,” the point at which voltage gated sodium channels in the axon hillock first begin to open and allow the influx of sodium, triggering an action potential
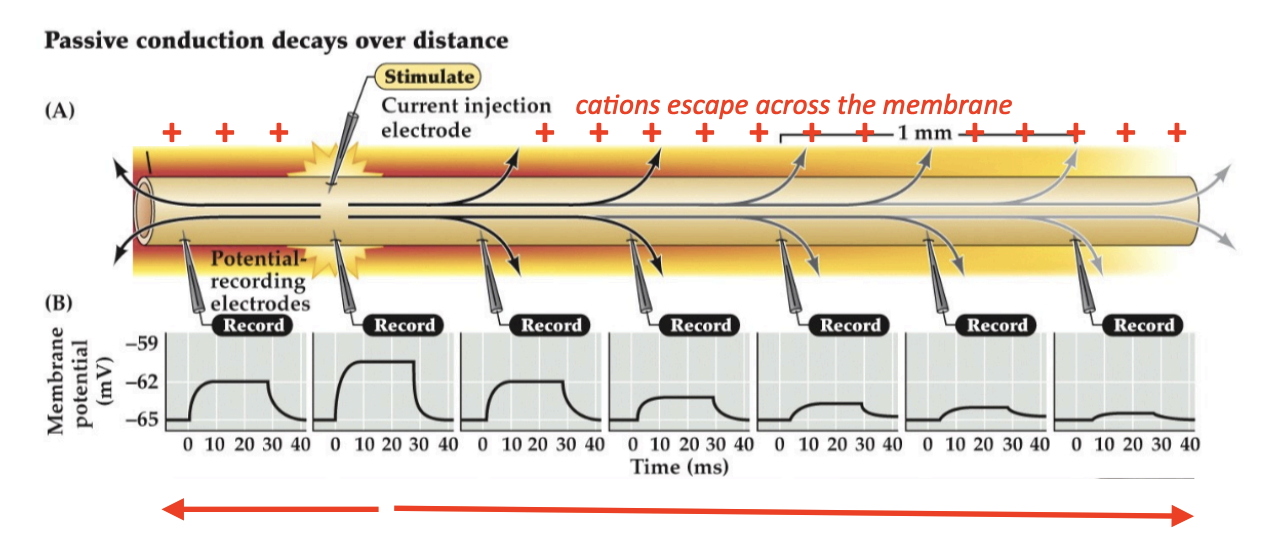
Passive conduction
Bidirectional, signal decays/weakens over distance from source
Active conduction
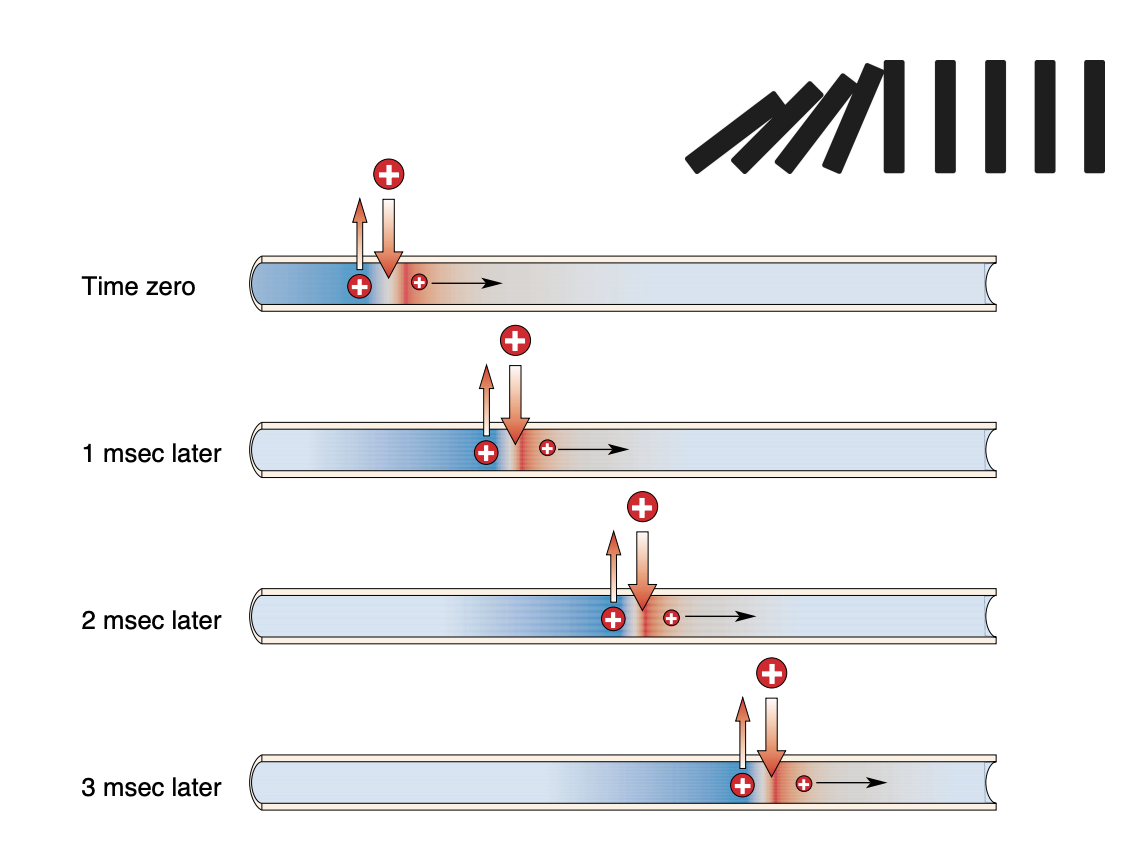
Action potentials don’t decay over distance because they’re regenerated by voltage-gated Na+ channels opening along the length of the axon; Na+ channel inactivation (refractory) in the wake of the AP ensures that APs only travel in one direction
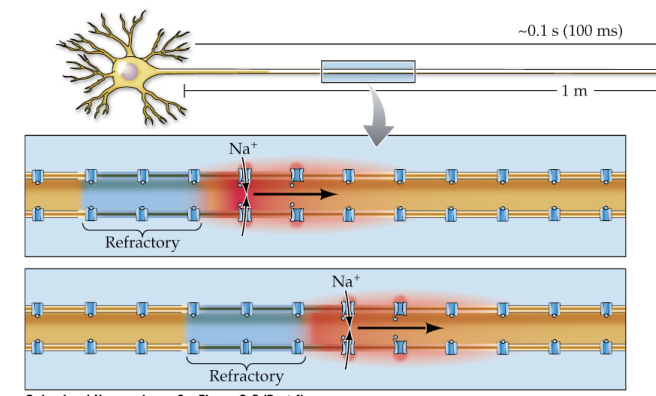
Action potential propagation
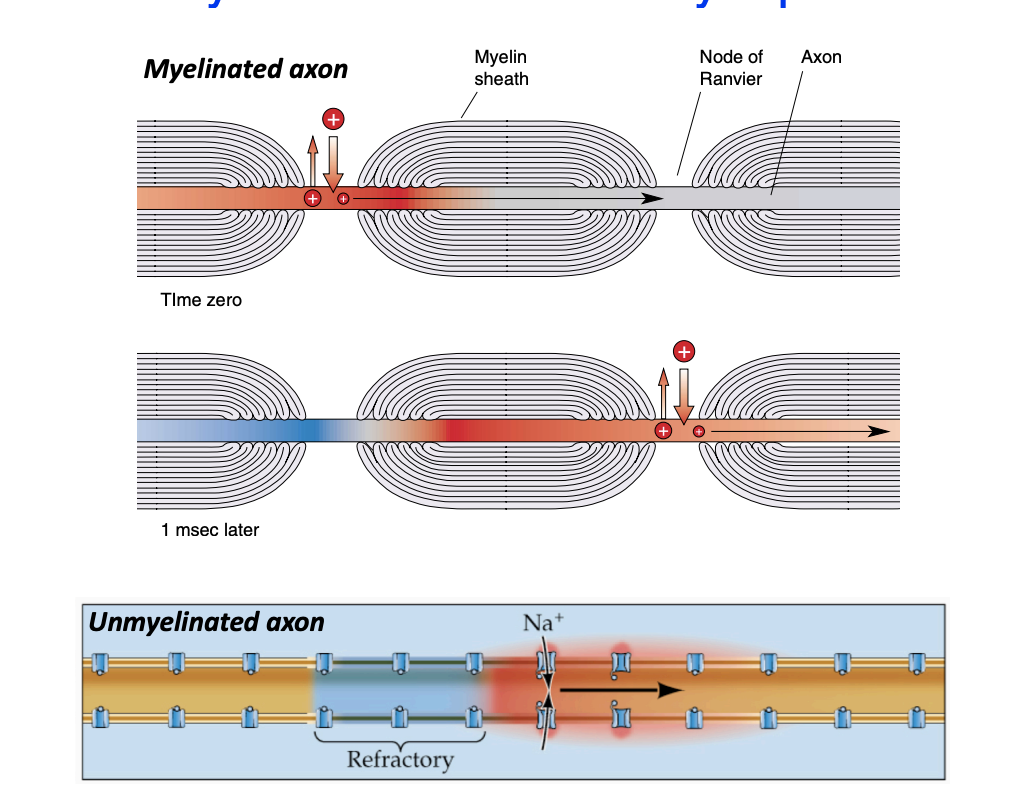
Nodes of Ranvier
small points along the axon where there’s no myelination and importantly, they have a high density of VGNa+ channels
Depolarization
This allows for a reduced axon diameter and dramatically reduces current/ion leakage
Having large diameter axons (e.g. the squid giant axon) is another way to increase conduction velocity (e.g. fire hose vs. garden hose) in the absence of myelination
Action potential regeneration
regenerated by the opening of VGNa+ Channels at Each Node of Ranvier
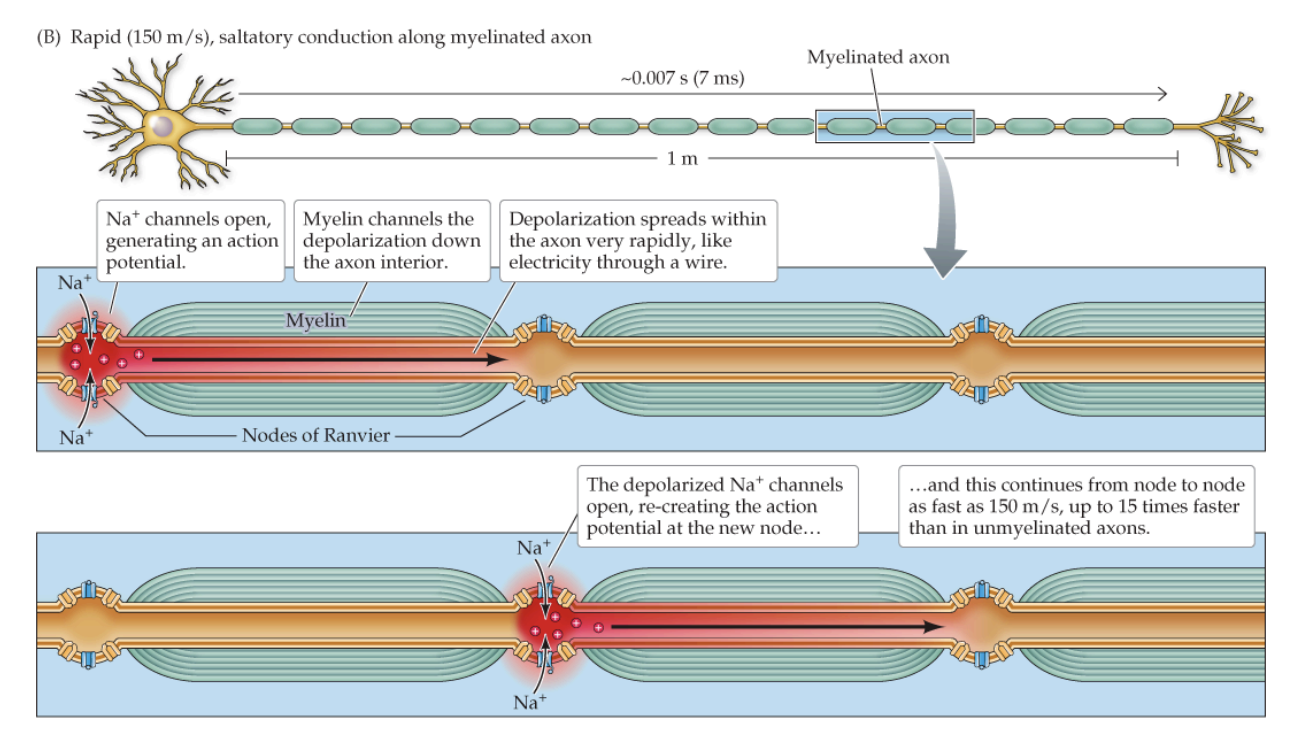
Saltatory conduction
Along myelinated axons
Very rapid
“jumps” from node to node, increasing conduction velocity (insulation from myelin reduces charge leakage out of the axon)
~150 m/s with myelin vs. 1-10 m/s without
Multiple sclerosis
demyelinating autoimmune disease that affects the brain, spinal cord, and peripheral neurons
loss of myelin in the nervous system slows AP conduction
Irregular, unpredictable step lengths and foot placement
Jerky, hesitant movements
Lack of deceleration and trunk control
Often requires use of upper extremities for support
Decreased safety
Electrode position
The farther the recording electrode is from the site of stimulation, the later the action potential reaches it, the size of the action potential is the same at each point along the axon.
What are voltage-gated sodium channels?
Specialized transmembrane proteins that open in response to changes in membrane potential.
They allow Na⁺ ions to rapidly enter the neuron, initiating the depolarization phase of the action potential.
Where on the neuron are voltage-gated sodium channels concentrated?
These channels are densely packed at the axon hillock (the initial segment of the axon) and along the Nodes of Ranvier in myelinated axons.
The axon hillock is the critical site for action potential initiation due to its high channel density.
Explain how action potentials propagate down the axon.
Once initiated, the depolarization spreads to adjacent segments of the axon.
This triggers nearby voltage-gated Na⁺ channels to open, creating a wave of depolarization down the axon.
How do voltage gated sodium channels open (or “activate”)?
At rest (~–70 mV), the activation gate is closed and the inactivation gate is open.
When the membrane potential reaches the threshold (~–55 mV), both gates open, allowing Na⁺ influx.
This influx causes rapid depolarization, pushing the membrane potential toward +30 mV.
How does voltage gated sodium channel activity relate to the concept of action potential “threshold?”
The threshold is the critical voltage at which enough Na⁺ channels open to trigger a self-propagating action potential.
Below threshold, the depolarization is insufficient to sustain channel opening; above it, the process becomes regenerative.
What role does myelination play in this process of action potential propagation?
Myelin sheaths (formed by oligodendrocytes in the CNS and Schwann cells in the PNS) insulate the axon, preventing ion leakage.
This enables saltatory conduction, where the action potential "jumps" from one Node of Ranvier to the next.
What are “Nodes of Ranvier?”
These are small gaps between myelinated segments where voltage-gated Na⁺ and K⁺ channels are concentrated.
They are essential for regenerating the action potential during saltatory conduction.
Why is it that action potentials do not “decay” as they propagate down the axon?
Unlike passive electrical signals, action potentials are actively regenerated at each node.
This prevents decay and ensures consistent signal strength over long distances.
Under normal physiological conditions, why can’t action potentials travel backwards, back up the axon toward the cell body? Hint: think about the properties of the voltage-gated sodium channel.
After activation, Na⁺ channels enter an inactivated state (activation gate open, inactivation gate closed).
This creates a refractory period, during which the channel cannot reopen, ensuring unidirectional propagation.