Inflammation and Shock - Foundations of Tissue Perfusion and Cellular Response
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
What is tissue perfusion?
The process of delivering oxygenated blood to tissues and removing waste products via the circulatory system
Importance of Tissue Perfusion
Maintains cellular metabolism and ATP production
Essential for organ function - brain, heart, kidneys are most sensitive
“muscles”
Impaired perfusion → ischemia, cell death, organ failure
“ischemia = inadequate oxygen supply”
What is the formula for cardiac output (CO)?
Cardiac Output (CO) = Stroke Volume x Heart Rate
Cardiac Output
Volume of blood pumped by the heart per minute (normal: 4-8 L/min)
Stroke Volume
Preload: ventricular filling (end-diastolic volume)
“end-diastolic volume = the amount of blood in the heart's ventricles (left and right) at the end of the relaxation phase (diastole)”
Contractility: Strength of ventricular contraction
Afterload: Resistance the ventricle must overcome to eject blood
Heart Rate
Controlled by autonomic nervous system:
Sympathetic: ↑HR (β1 receptors)
“Fight or Flight, ↓SV”
Parasympathetic: ↓HR (vagus nerve)
“Rest and Digest, ↑SV”
Definition of Systemic Vascular Resistance (SVR)
Resistance blood encounters in systemic circulation (mainly arterioles)
“blood → get to tissues”
“arterioles = small branch of an artery leading into capillaries”
Analogy of Systemic Vascular Resistance (SVR)
SVR acts like a vise grip on the aorta - tight grip = harder for blood to flow
Impact of Systemic Vascular Resistance (SVR)
↑SVR → ↑Afterload → ↑Cardiac workload
“vasoconstriction”
↓SVR → ↓Afterload → easier ejection but risk of hypotension
“vasodilation”
“during hypotension: fluid is hanging out in the tissues, not going back to the heart”
Venous System
Stores 70-80% of total blood volume (acts as reservoir)
Venous Return
Influences preload and therefore stroke volume
Regulation of Venous Return & Blood Reservoir
Vasoconstriction: ↑Venous return → ↑Preload → ↑CO
Vasodilation: ↓Venous return → ↓Preload → ↓CO
What is Inflammation?
A protective response to injury, infection, or irritation
Goals of Inflammation
Eliminate the initial cause of cell injury
Clear out necrotic cells and tissues
Initiate tissue repair
Clinical Relevance of Inflammation
Seen in infections, trauma, autoimmune conditions
Acute Inflammation
Rapid onset, short duration
Predominantly neutrophil response
Examples: Appendicitis, cellulitis
Chronic Inflammation
Persistent, long-term
Involves macrophages, lymphocytes
Examples: Rheumatoid arthritis, Crohn’s disease
Cardinal Signs of Inflammation
Redness (rubor)
Heat (calor)
Swelling (tumor)
Pain (dolor)
Loss of function (functio laesa)
Cardinal Signs of Inflammation: Redness (rubor)
Due to vasodilation and increased blood flow
Cardinal Signs of Inflammation: Heat (calor)
Increased blood flow and metabolic activity
Cardinal Signs of Inflammation: Swelling (tumor)
Accumulation of fluid from increased vascular permeability
Cardinal Signs of Inflammation: Pain (dolor)
Release of chemical mediators stimulating nerve endings
Cardinal Signs of Inflammation: Loss of function (functio laesa)
Result of pain and swelling limiting movement
Cellular & Chemical Mediators: Neutrophils
First responders, phagocytose pathogens
Cellular & Chemical Mediators: Macrophages
Clean up debris, release cytokines
“cytokines → big inflammatory mediators, raise temperature”
Cellular & Chemical Mediators: Mast Cells
Release histamine, trigger vasodilation
“seen earlier in inflammatory processes”
Cellular & Chemical Mediators: Cytokines
IL-1, TNF-alpha promote inflammation and fever
Cellular & Chemical Mediators: Histamine
Increases vascular permeability
Cellular & Chemical Mediators: Prostaglandins
Mediate pain and fever
What are the systemic effects of inflammation?
Fever
Leukocytosis
Increased CRP and ESR
Link to SIRS
Systemic Effects of Inflammation: Fever
Triggered by cytokines acting on hypothalamus
Systemic Effects of Inflammation: Leukocytosis
↑ Elevated white blood cell count
Systemic Effects of Inflammation: Increased CRP and ESR
Markers of systemic inflammation
Systemic Effects of Inflammation: Link to SIRS
Systemic inflammatory response can progress to septic shock
“SIGNS: fever, tachycardia, hypotension, decreased urine output”
Inflammation & Shock Connection
Inflammatory mediators cause vasodilation and increased capillary permeability
Fluid shifts from intravascular to interstitial space
↓Preload → ↓Cardiac output → ↓Tissue perfusion
Seen in septic shock and other distributive shock states
Distributive Shock
Often results from systemic inflammation
(e.g., sepsis, anaphylaxis)
Cytokine Storm
Excessive release of inflammatory cytokines
(e.g., IL-1, TNF-alpha)
“seen in autoimmune diseases”
Effects of Inflammation (Cytokine Storm) and (Distributive) Shock
Vasodilation
Increased capillary permeability
Fluid shifts → ↓Preload and ↓Perfusion
What is Stress Hyperglycemia triggered by?
Critical illness, trauma, or infection
What are the hormonal drivers of Stress Hyperglycemia?
Catecholamines, cortisol, glucagon → ↑gluconeogenesis and insulin resistance
What is the clinical impact of Stress Hyperglycemia?
Hyperglycemia can impair immune function and wound healing
What are the blood glucose targets in a patient with Stress Hyperglycemia?
140-180 mg/dL in critically ill patients (per ADA guidelines)
Clinical Pearls: What does Stroke Volume (SV) depend on?
Preload
Contractility
Afterload
Clinical Pearls: Pump vs. Volume
Evaluate both myocardial function and intravascular volume status
“Heart innate function vs. fluid status”
Definition of Shock
A life-threatening condition where the circulatory system fails to deliver adequate oxygen and nutrients to meet tissue metabolic demands
Common Pathophysiologic Thread of Shock
Inadequate tissue perfusion → cellular hypoxia → anaerobic metabolism → lactic acidosis → organ dysfunction
Key Consequences of Shock
↓ATP production
Cell membrane dysfunction
Multi-organ failure if untreated
Cause of Hypovolemic Shock
Absolute fluid loss: hemorrhage (trauma, GI bleed), severe dehydration
Relative fluid loss: Third spacing (burns, peritonitis)
Pathophysiology of Hypovolemic Shock
↓Intravascular volume → ↓Venous return (preload) → ↓Stroke volume → ↓Cardiac output → ↓Tissue perfusion
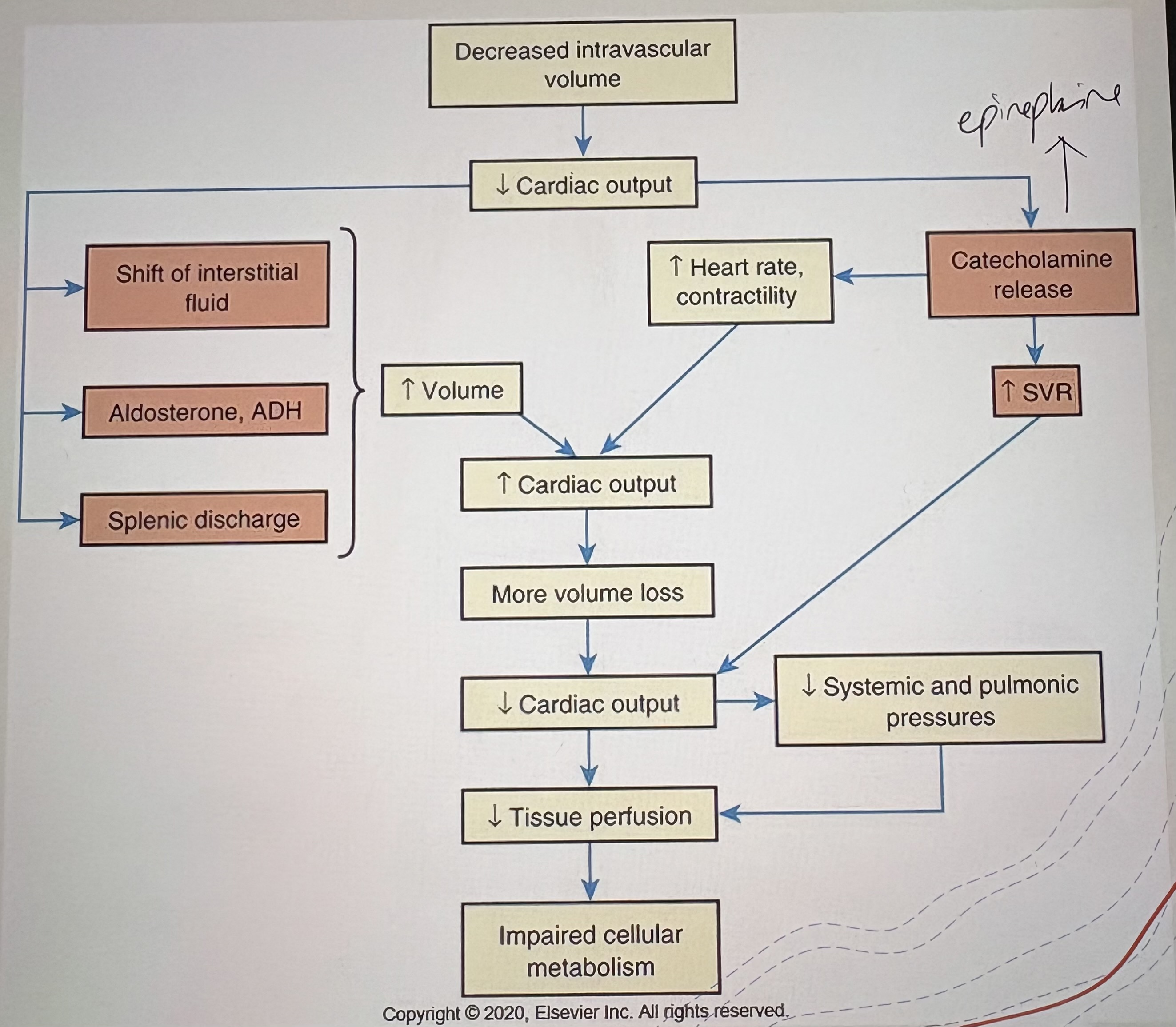
Clinical Clues of Hypovolemic Shock
Tachycardia
Hypotension
Cool, clammy skin
“due to vasoconstriction”
↓ Urine output
Cause of Cardiogenic Shock
Myocardial infarction (most common)
Severe heart failure
Arrhythmias
Pathophysiology of Cardiogenic Shock
Pump failure → ↓Stroke volume despite adequate volume → ↓Cardiac output → ↓Tissue perfusion
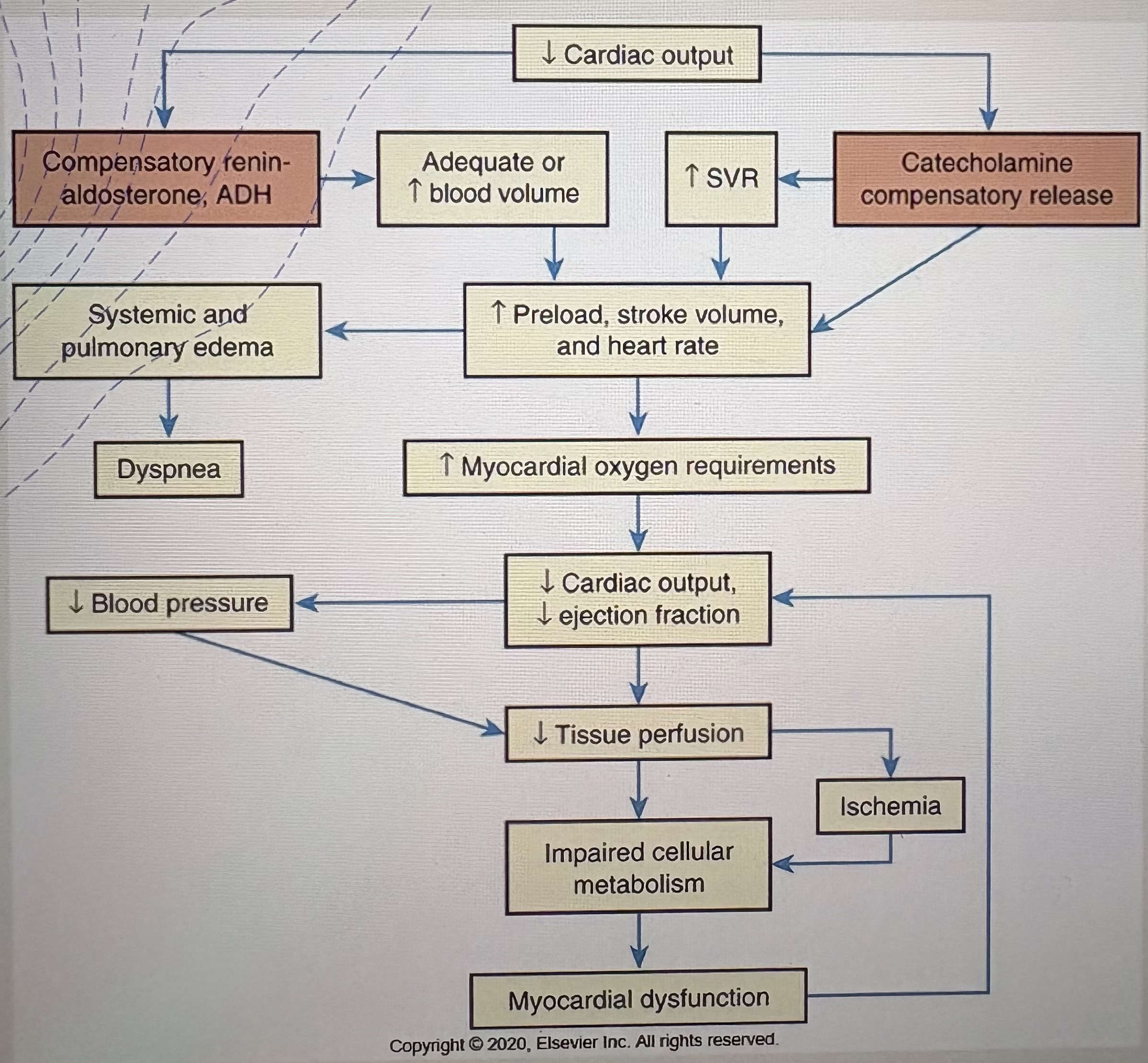
Clinical Clues of Cardiogenic Shock
Pulmonary edema
“fluid in lungs → swelling”
Jugular venous distension
Hypotension
Weak pulses
Cause of Obstructive Shock
Cardiac tamponade
Massive pulmonary embolism
Tension pneumothorax
Pathophysiology of Obstructive Shock
Physical obstruction to blood flow → ↓Venous return or ↓Outflow → ↓Cardiac output → ↓Tissue perfusion
Clinical Clues of Obstructive Shock
Distended neck veins
Muffled heart sounds (tamponade)
Sudden dyspnea (PE)
Subtypes of Distributive Shock
Septic
Neurogenic
Anaphylactic
Pathophysiology of Distributive Shock
Massive vasodilation → ↓SVR → relative hypovolemia → ↓Tissue perfusion
Cause of Septic Shock
Severe infection leading to systemic inflammatory response
Pathophysiology of Septic Shock
Inflammatory mediators → vasodilation and capillary leak → ↓SVR and preload → ↓Tissue perfusion
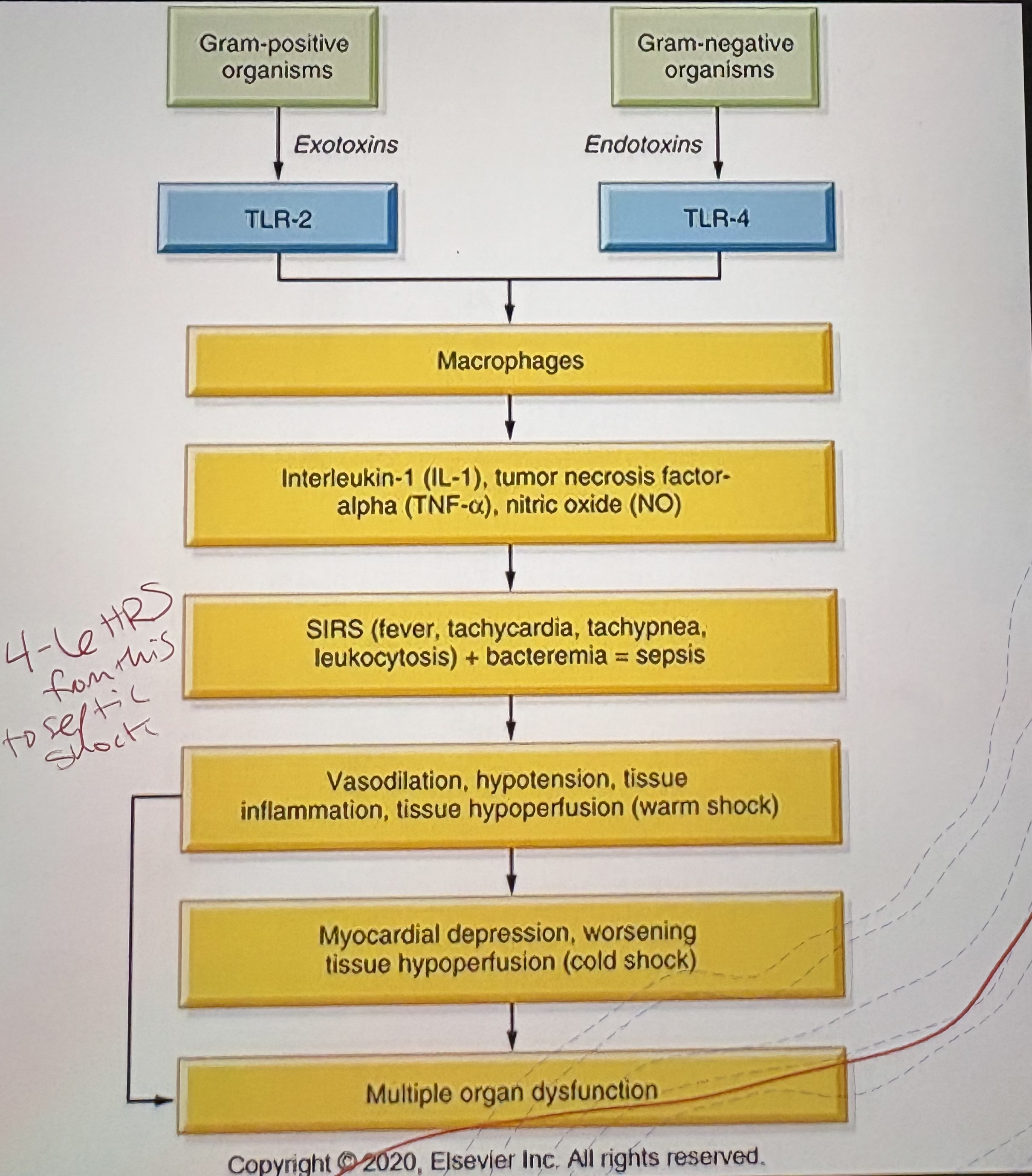
Clinical Features of Septic Shock
Fever
Warm, flushed skin (early)
Hypotension
Tachycardia
Cause of Neurogenic Shock
Spinal cord injury disrupting sympathetic tone
Pathophysiology of Neurogenic Shock
Loss of sympathetic tone → vasodilation → ↓SVR → ↓Tissue perfusion
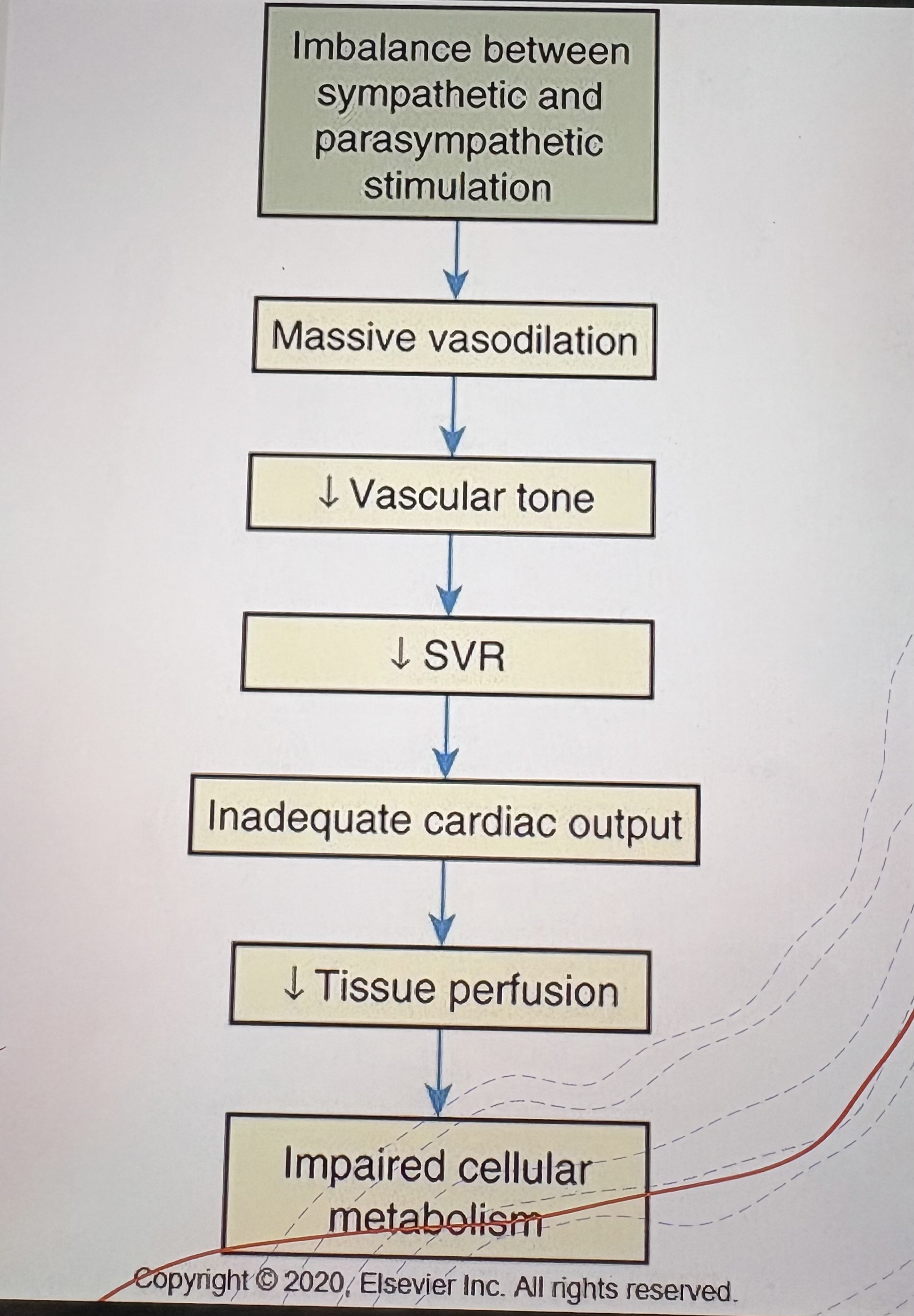
Clinical Features of Neurogenic Shock
Bradycardia
Hypotension
Warm, dry skin
Cause of Anaphylactic Shock
Severe allergic reaction (e.g., food, insect stings, medications)
Pathophysiology of Anaphylactic Shock
Histamine release → massive vasodilation and increased capillary permeability → ↓SVR and preload → ↓Tissue perfusion
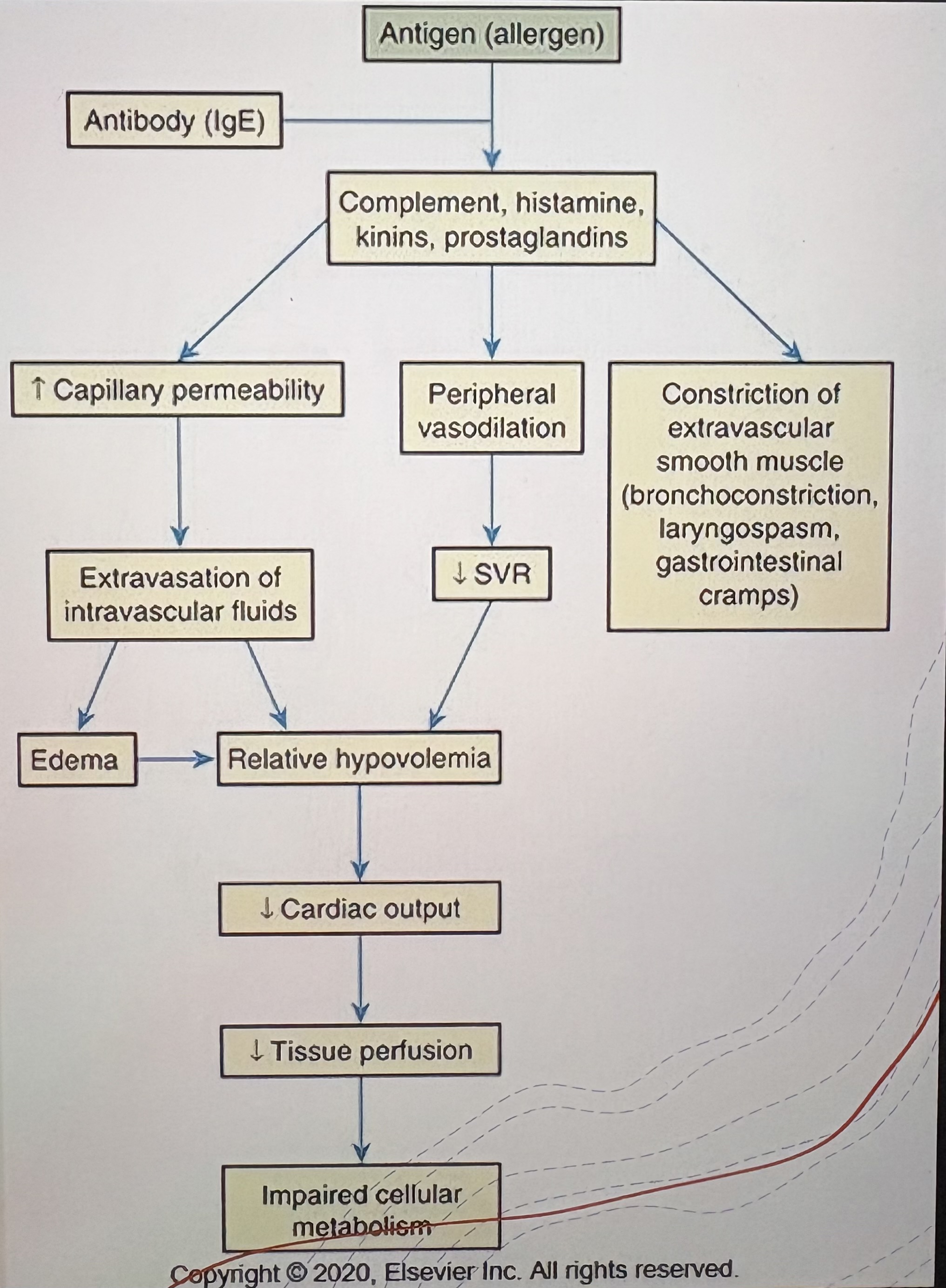
Clinical Features of Anaphylactic Shock
Hypotension
Tachycardia
Airway compromise
Urticaria
Anaerobic Metabolism in Shock
In shock, reduced oxygen delivery forces cells into anaerobic glycolysis
Anaerobic metabolism yield only 2 ATP per glucose (vs. 36 in aerobic)
Lactic acid accumulates → metabolic acidosis → impaired cellular function
What are the clinical signs of anaerobic metabolism in shock?
Elevated lactate
low pH
Tachypnea
Mitochondrial Dysfunction
Mitochondria are essential for ATP production via oxidative phosphorylation
In shock, hypoxia and toxins impair mitochondrial function
Analogy: ‘Canaries in the coal mine’ - mitochondria fail early in stress
Consequences of Mitochondrial Dysfunction
↓ATP → cell death, organ failure
Electrolyte Derangements
Na+/K+ pump failure due to ATP depletion
Sodium and water enter cells → cellular edema
Potassium leaks out → hyperkalemia
Membrane permeability increases → risk of cell lysis
Why CRP Increases in Shock States
CRP (C-reactive protein) is an acute-phase reactant made by the liver
Triggered by cytokines like IL-6 during systemic inflammation
Shock (septic, cardiogenic, hypovolemic) causes tissue hypoxia and injury
Cytokine release stimulates CRP production
CRP binds to damaged cells, activates complement, and enhances phagocytosis
Clinical Significance of Increased CRP in Shock States
Elevated CRP reflects inflammation and tissue damage
In septic shock, CRP correlates with severity and prognosis
Normal: <10 mg/L | Moderate: 10-100 mg/L | Severe: >100 mg/L
What are normal lactate levels?
0.5-2.2 mmol/L
What do elevated levels of serum lactate indicate?
They indicate tissue hypoxia and anaerobic metabolism
What is the prognostic marker for serum lactate?
Higher levels correlate with worse outcomes
What is serum lactate as a marker used for?
Used to guide resuscitation and monitor response to therapy
Procalcitonin (PCT)
Precursor of calcitonin, elevated in bacterial infections
Rises early in sepsis and septic shock
Helps differentiate bacterial vs. viral infections
Levels > 2 ng/mL suggest severe systemic infection
D-dimer
Fibrin degradation product, elevated in coagulation activation
Increased in septic shock, DIC, and thromboembolic events
Non-specific but useful in ruling out PE/DVT
High levels may indicate poor prognosis