Exam 4 - BIOL 3200 - Riggs
1/151
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
152 Terms
Slideshow 1: Antimicrobial Therapy
What did Ernest Duchesne propose?
bacteria and molds engage in a perpetual battle for survival
What did Alexander Fleming discover?
penicillin from Penicillium notatum
Who purified penicillin?
Howard Florey and Ernst Chain
What did Gerhard Domagk discover?
- sulfa drugs (prontosil dye) which is inactive until converted by the body
- analogs of PABA, a precursor of vitamin for DNA synthesis
What did Selman Waksman discover?
Streptomycin, antibiotic produced by actinomycete bacterium found in the soil (Streptomyces griseus)
name and describe the 2 fundamentals of antimicrobial therapy
1. must affect target organism (PTG, diff ribosome structure, diff biochemical pathway)
2. can't affect human (some have side effects at high conc)
-- chloramphenicol interferes with RBC development
name and describe the 2 spectrums of activity
- broad: effective against many species (G+ and G-)
- narrow: Isoniazid only works on Mycobacterium tuberculosis
minimal inhibitory concentration (MIC) overview
- lowest concentration that prevents growth
- may still have living but non-growing organisms
What does it mean if a MIC tube is plated and no colonies are present?
- minimal lethal concentration (MLC or MBC)
- MLC is always higher conc than MIC
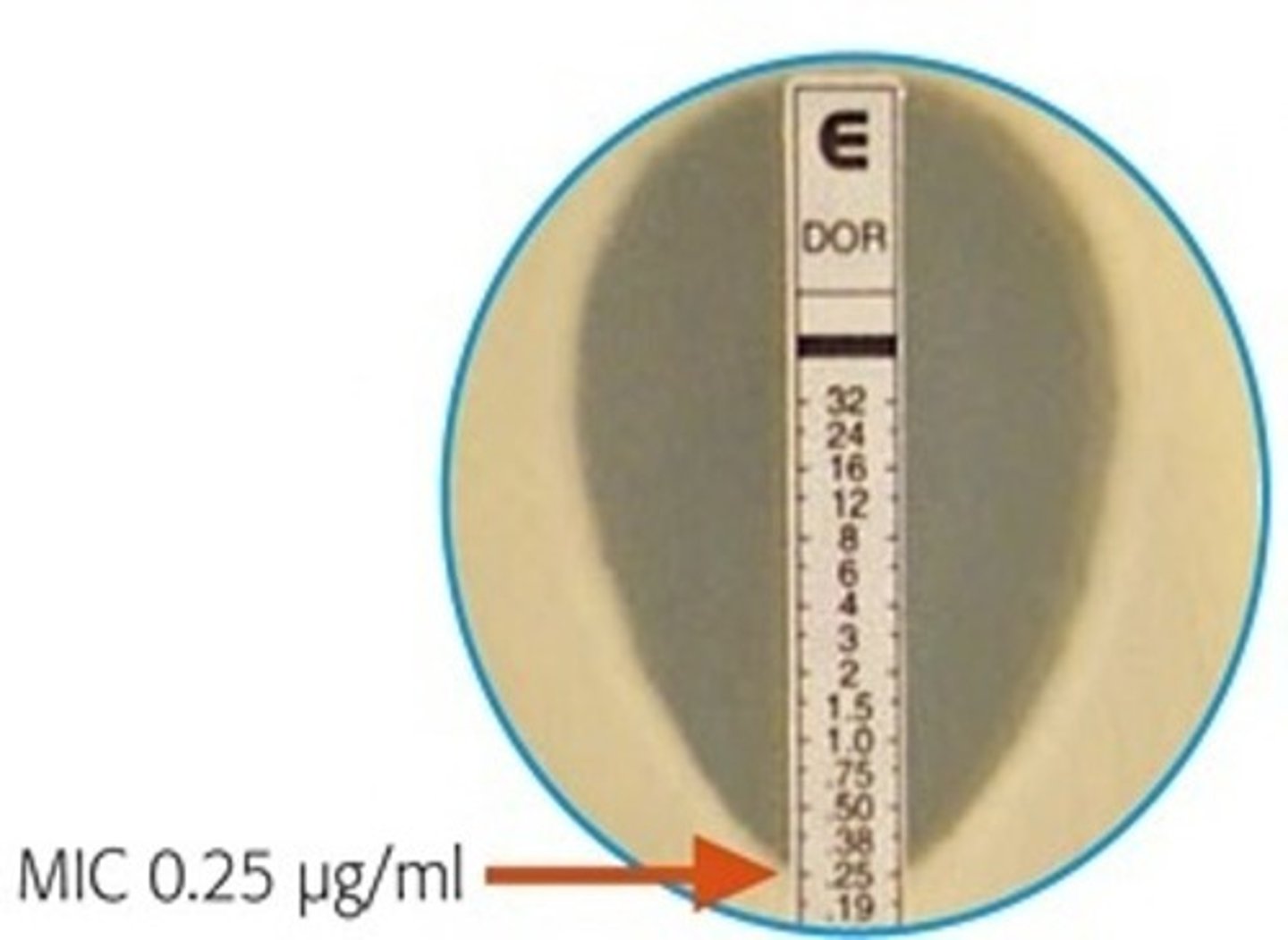
What is a MIC strip test?
- no need for dilutions, reduces time to determine effectiveness
- the MIC is the point at which the elliptical zone of inhibition intersects with the strip
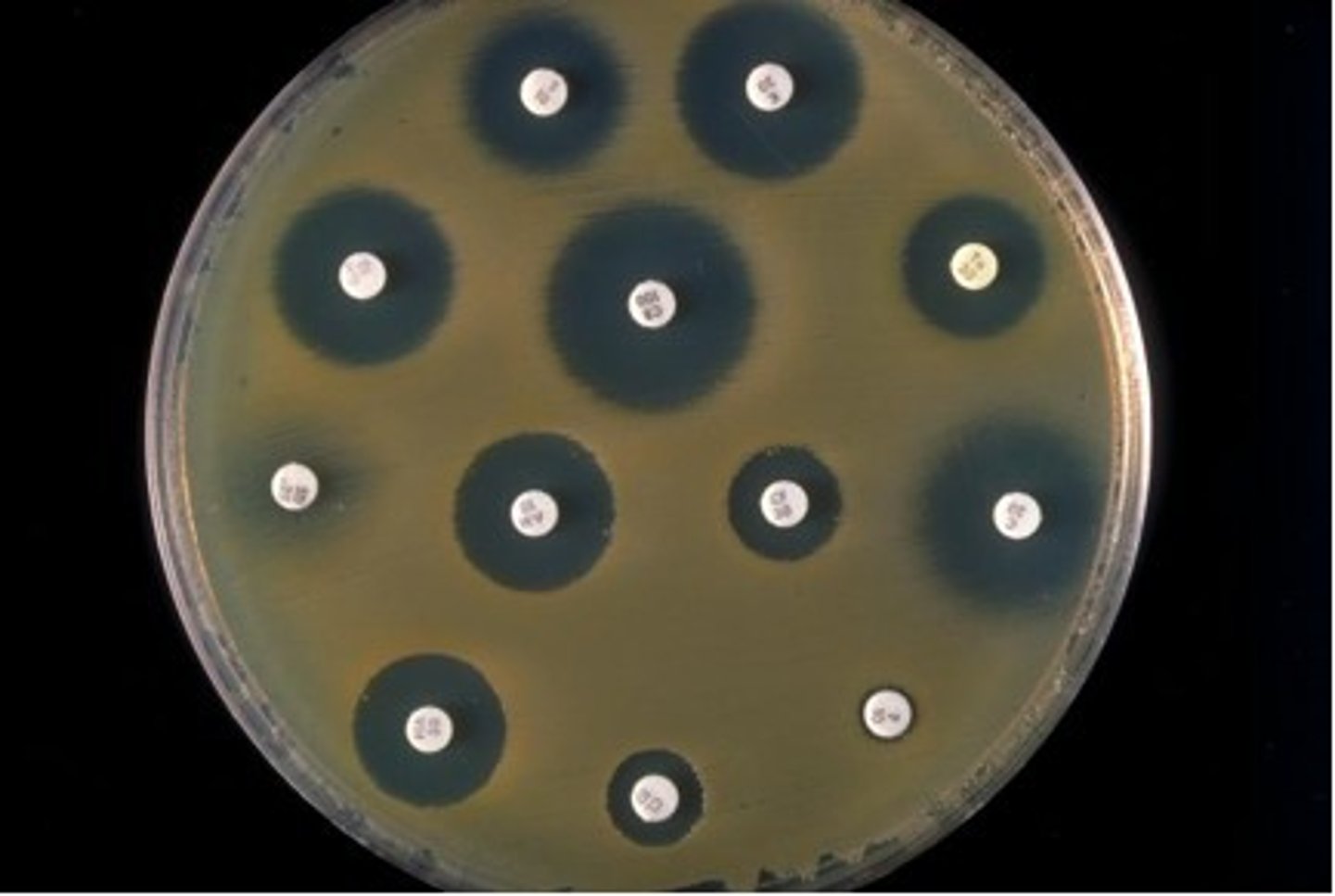
Kirby-Bauer disk susceptibility test
- easier testing
- size of clear zone reflects relative sensitivity
what does penicillin target?
cell wall
what does rifamycin B target?
RNA synthesis
what does polymyxin target?
cell membrane
What does gramicidin target?
cell membrane
what do aminoglycosides target?
protein synthesis
what does quinolones target?
DNA synthesis
What does vancomycin target?
cell wall
What does actinomycin D target?
RNA synthesis
what do sulfa drugs target?
DNA synthesis
what does tetracycline target?
protein synthesis
cell wall antibiotics: explain PTG synthesis and what penicillin and cephalosporins do to this?
- PTG synthesis:
1. precursors UDP-NAG and UDP-NAM-peptide are made in cytoplasm
2. carried across cell membrane by lipid carrier bactoprenol
3. precursors are polymerized to the existing cell wall structure by transglycosylases
4. the peptide side chains are cross-linked by transpeptisidases
- The cross-bridge formation by transpeptidase is blocked by penicillin and Cephalosporins
Beta-Lactam antibiotics
- penicillins and cephalosporins which contain beta-lactam ring
-- this BL ring chemically resembles the D-Ala-D-Ala piece of PTG
-- this mimicry allows drug to bind to penicillin-binding proteins (transpeptidase and transglycosylase) preventing their activities and stopping synthesis
-- R groups can be modified to generate a number of semisynthetic drugs
name and describe the 3 penicillin mechanisms of action
- natural penicillin
-- narrow spectrum
-- effective against G+ and some G- cocci
- penicillinase-resistant penicillin
-- side chains prevent inactivation by penicillinase enzymes
- broad-spectrum penicillin
-- effective against G+ and G-
vancomycin
- affect cell wall synthesis
- very large and complex glycopeptide produced by Streptomycete spp.
- binds to D-Ala-D-Ala terminal and prevent action of transpeptidases and transglycosylases (whereas penicillin binds to transpeptidases and transglycosylases instead)
- doesn't cross LPS outer membrane of G-
- must be given IV bc of poor GI absorption
gramicidin
- disrupt cell membrane
- Cyclic peptide produced by Bacillus brevis
- Forms a cation channel, through which ions leak
polymyxin (colistin)
- disrupt cell membrane
- Produced by Bacillus polymyxa
- Destroys cell membrane, just like a detergent
- Used only topically
quinolones (nalidixic acid and ciprofloxacin)
- affect DNA synthesis and integrity
- Block bacterial DNA gyrase, and so prevent DNA replication
- Bactericidal
Sulfa drugs (sulfonamides)
- affect DNA synthesis and integrity
- Analogs of PABA, a precursor of folic acid
-- Needed for DNA synthesis
-- folic acid is supplied in human diet; no folic acid synthesis to inhibit in humans, therefore, selectively toxic due to competitive inhibition of folic acid synthesis enzymes
sulfa/folic
antibiotics that inhibit transcription are _______ and most active against _______ bacteria.
bactericidal, growing
rifamycin B (rifampin)
- inhibits RNA synthesis
- Binds to the beta subunit of RNA polymerase
- Prevents the elongation step of transcription
B/beta
actinomycin D
- inhibits RNA synthesis
- prevents initiation and elongation step of transcription
- binds to DNA from any source (not selective and is toxic to host)
aminoglycosides (streptomycin)
- inhibit protein synthesis, affect 30S subunit
- cause translational misreading of mRNA
- bactericidal
- have cyclohexane ring and amino sugars
tetracyclines
- inhibit protein synthesis, affect 30S subunit
- block binding of charged tRNAs to the A site
- bacteriostatic
- include doxycyline
- have four-ring structures to which a variety of side chains attach
TetrAcycline/tRNA at A site
erythromycin
- inhibit protein synthesis, affect 50S subunit
- inhibit translocation
erythroMycin/Movement/translocation in translation
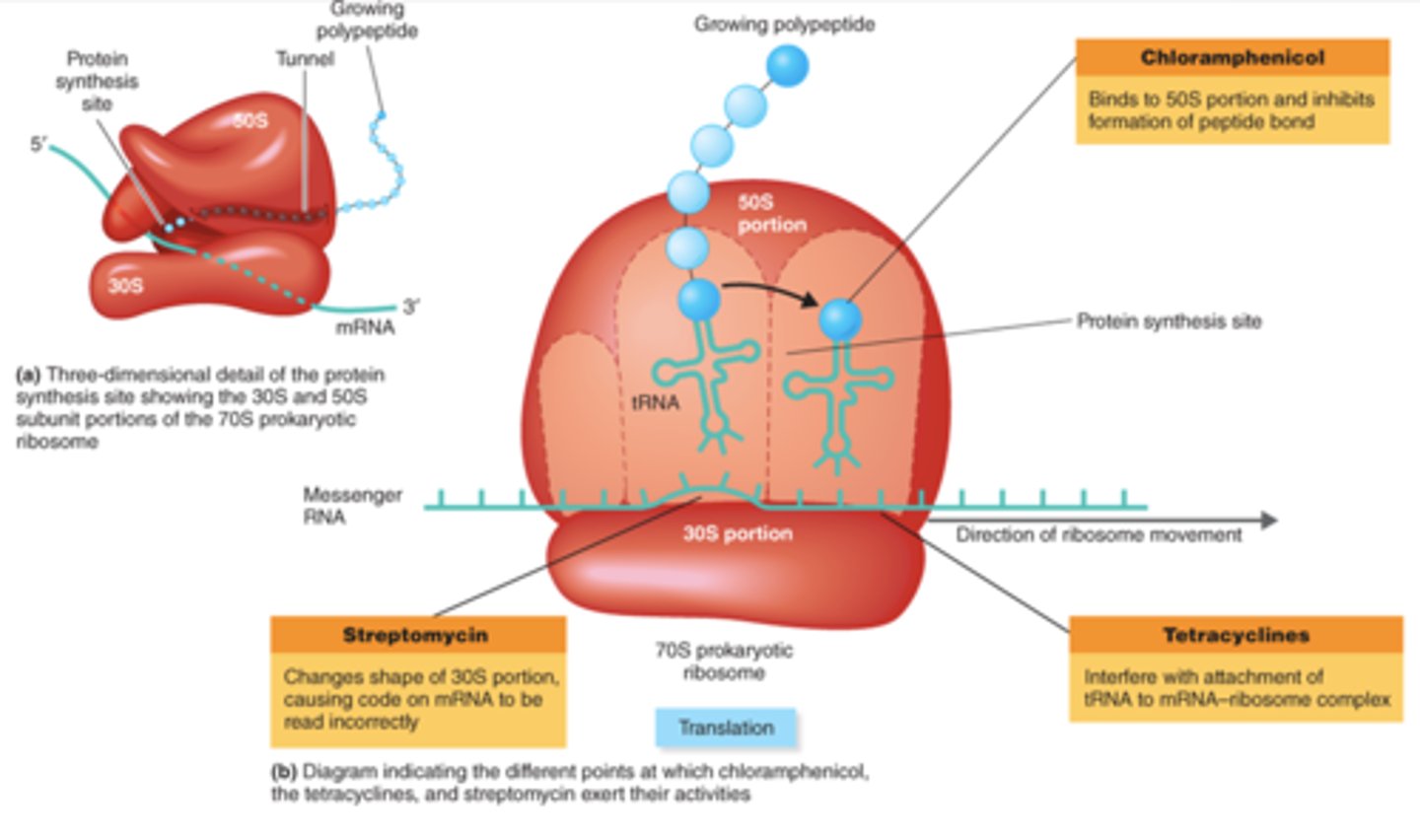
chloramphenicol
- inhibit protein synthesis, affect 50S subunit
- inhibit peptidyl transferase activity
- only used in life threatening situations bc its toxic with numerous side effects
clindamycin and metronidazole
- inhibit protein synthesis, affect 50S subunit
- bind at same ribosomal site as chloramphenicol
- active in anaerobic environments
protein synthesis inhibitors picture
what is the common cold cause by?
rhinovirus, no antibiotic is designed for bacteria to touch it
Why are there so few antiviral agents?
- applying the principle of selective toxicity is much harder for viruses than it is for bacteria
- viruses seize host cell functions to make copies of themselves
antiviral agents that prevent virus uncoating or release
- influenza virus which is enveloped is vulnerable
-- amantadine inhibits viral uncoating, preventing entry of virus into host cell, no longer used bc of resistance
- neuraminidase inhibitors
-- prevents release of mature viruses
-- zanamivir and oseltamivir (tamiflu)
how do most antivirals work?
- by inhibiting viral DNA synthesis
- resemble normal DNA nucleosides but lack 3' OH, and so cause chain termination
- inserted into growing viral DNA strand, inhibit new DNA synthesis
are fungal infections easier or harder to treat than bacterial infections? why or why not?
- harder
- fungi are eukaryotes so selective toxicity issues arise
- fungi have an efficient drug detox system that modifies and inactivates many drugs
What two groups can fungal infections divide into?
- superficial mycoses: treated topically
- deep mycoses: treated systemically
name the 4 antifungal agents
synthetic: azoles, terbinafines
produced by microorganisms: polyenes, griseofulvin
antifungal agent: azoles
- inhibit the synthesis of membrane sterols
- Synthetic drugs
- Clotrimazole, itraconazole, fluconazole
azoles/sterols
antifungal agent: terbinafines
- selectively inhibit ergosterol synthesis
- Synthetic drugs
- Humans do not make ergosterol nor do they use it in their cell membranes.
terbin/ergo
antifungal agent: polyenes
- form pores in fungal cell membranes
- Produced by Streptomyces species
- Amphotericin B, Nystatin
poly/pores
antifungal agent: griseofulvin
- disrupts the mitotic spindle
- Produced by Penicillium species
i in griseofulvin looks like a rod, spindle means rod
Slideshow 2: Antimicrobial Resistance
what did Paul Ehrlich develop and identify?
- developed concept of selective toxicity
- identified dyes that effectively treated African sleeping sickness
what did Sahachiro Hato identify?
identified arsenic compounds that effectively treated syphilis. worked with Ehrlich
what did Domagk, Jacques, and Trefouel discover?
sulfonamides/sulfa drugs
penicillin timeline
- "discovered" by Ernest Duchesne (talked about penicillium mold inhibiting growth)
- re-discovered by Alexander Fleming (actually saw isolated penicillium inhibit growth)
- effectiveness demonstrated by Florey, Chain, and Heatley, who won Nobel Prize
Who discovered streptomycin? what did this do?
Selman Waksman, opened door for many more antibiotics produced by soil bacteria
what is selective toxicity?
ability of a drug to kill or inhibit pathogen while damaging host as little as possible
what is the therapeutic dose?
drug level required for clinical treatment
What is the toxic dose?
drug level at which drug becomes too toxic for patient
what is the therapeutic index?
ratio of toxic dose to therapeutic dose
can zone of inhibition width be used to compare antibiotic's effectiveness? why or why not?
- textbook answer: no, it is a function of antibiotic concentration, solubility, and diffusion rate
- chatgpt explanation: You cannot compare different antibiotics by zone width alone because some diffuse at diff rates and are of higher conc in the disk. You can compare how one bacterium responds to one antibiotic based on whether the zone size meets a threshold for susceptibility.
What do many penicillin resistant organisms produce and what does this do against penicillin?
produces B-lactamase (penicillinase) which hydrolyzes a bond in the B-lactam ring
what is the difference between naturally occurring penicillins, semisynthetic penicillins, and aminopenicillins?
- natural: P V and G are narrow spectrum
- semisynthetic: broader spectrum with bulkier side chains making them more difficult for penicillinase to degrade
- aminopenicillins have broader coverage including G-
In what situation would cephalosporins be used instead of penicillin?
if pt is allergic to penicillin
what is vancomycin important in treating and what is it considered?
- treating antibiotic-resistant staphylococcal and enterococcal infections
- considered drug of last resort so rise in resistance is concerning
What are the protein synthesis inhibitors which binds to bacterial ribosomal proteins or rRNA?
- aminoglycosides
- tetracyclines
- macrolides (erythromycin)
- lincosamides (clindamycin)
- chloramphenicol
CLAMT
macrolides
- contain 12-22 C lactone ring linked to one or more sugars
- example being erythromycin
- can be used for pt allergic to penicillin
lincosamides
- produced by Streptomyces bacteria
- broad spectrum against anaerobic microbes, less against aerobic
- used sparingly bc they can indirectly support the growth of C. diff which brings more disease
- example being Clindamycin
What do metabolic antagonists act like and what are they?
- act like antimetabolites
-- antagonize or block functioning of metabolic pathways by competitively inhibiting the use of metabolites by key enzymes
- are structural analogs
-- structurally similar to and compete with naturally occurring metabolic intermediates to block normal cellular metabolism
trimethoprim
- synthetic antibiotic that interferes with folic acid production along with sulfa drugs
- broad spectrum
- can be combined with sulfa drugs to inc efficacy of treatment, blocking 2 steps in folic acid pathway
name the 2 most commonly used nucleic acid synthesis inhibitors and how they function?
- fluoroquinolones: inhibit DNA gyrase and topoisomerases
- rifamycins: inhibit RNA polymerase
not as selectively toxic as other antibiotics bc bacteria and eukaryotes somewhat synthesize NA similarly
fluoroquinolones
- synthetic, contain 4-quinolone ring
- act by inhibiting bacterial DNA gyrase and topoisomerase IV
- broad spectrum, bactericidal, treat wide variety of infections
intrinsic drug resistance
- occurs due to a property of the microbe itself
- mycoplasma resistance to B-lactam antibiotics and other cell wall inhibitors simply bc these bacteria lack a cells wall
acquired drug resistance
occurs when there is a change in the genome of a bacterium that converts it from one that is sensitive to an antibiotic to one that is resistant
drug-tolerant bacteria (persisters)
lack the mechanisms for antibiotic resistance and "ignore" the presence of antibiotics, usually because they are embedded in biofilms that antibiotics cannot effectively penetrate or are growing too slowly to be inhibited.
what are the challenges of drug resistance?
- antibiotics are secondary metabolites bc they have no use in producing an organism
-- not essential for survival but enhance ability to survive competition
- microbes can prevent self-destruction by means of various antibiotic resistance mechanisms
-- ex: make enzymes to disable antibiotics
-- genes encoding some of these drug-resistance mechanisms have been transferred to pathogens
what are the 4 basic forms of antibiotic resistance?
1. modify the target of the antibiotic
2. drug inactivation
3. minimize the conc of antibiotic in the cell, pump antibiotic out of the cell
4. bypass the chemical reaction inhibited by the agent or inc the production of the target metabolite
antibiotic resistance genes can be ____-____ or they can be part of the ____
plasmid-borne, chromosome
what is an example of the 1st mechanism of antibiotic resistance? "modify the target so that it no longer binds the antibiotic"
mutations in ribosomal proteins confer resistance to streptomycin
explain the 2nd mechanism of antibiotic resistance "destroy the antibiotic before it gets into cell
- production of B-lactamase enzyme specifically destroys penicillins and cephalosporins
- penicillinase attacks natural penicillins
explain the 3rd mechanism of antibiotic resistance "add modifying groups that inactivate the antibiotic"
3 types of enzymes modify and inactivate the aminoglycoside antibiotics (gentamicin)
antibiotic resistance mechanism 4: pump antibiotic out of the cell
- specific and nonspecific transport proteins
- efflux pumps can pump a wide range of drugs
what are the 2 mentioned drug-resistant strains?
- Streptococcus pneumoniae
- Acinetobacter baumanii which is resistant to multiple durgs
how does drug resistance develop?
- from the beginning antibiotic resistance develops through gene duplication and/or mutations (vertical evolution)
- can be acquired via HGT: conjugation, transduction, and transformation
how to overcome drug resistance
- appropriate conc
- 2 or more drugs at same time
- only when necessary
- possibility of new drugs in future or bacteriophages
intriguing ideas that may lead to novel antimicrobial therapies
1. nanotubes to poke holes in bacterial cell membranes
2. molecules that "cork" the type III secretion apparatus
3. interfering with the quorum-sensing mechanisms
Slideshow 3: Conjugation, Transformation, and Transduction
what did J. Lederberg and E. Tatum demonstrate?
the transfer of genes bw bacteria that depends on direct cell to cell contact mediated by F pilus and unidirectional DNA transfer from donor to recipient
Who demonstrated transformation?
Frederick Griffith
explain transformation
only recipient cell involved, uptake of naked DNA by a competent cell followed by incorporation of the DNA into the recipient cell's genome
explain transduction
done by viruses (bacteriophage) which can carry out the lytic cycle (host cell destroyed) or viral DNA integrates into the host genome becoming a latent prophage
generalized transduction
- any part of bacterial genome can be transferred (in the word "generalized")
- occurs during lytic cycle of virulent phage
- during viral assembly, fragments of host DNA are mistakenly packaged into phage head
-- generalized transducing particle
immunity genes and HGT
- immunity genes: resistance genes that exist in nature to protect antibiotic producing microbes from their own antibiotics
- HGT: transferred immunity genes from antibiotic producers to non-producing microbes
where can resistance genes be found?
- bacterial chromosomes
- plasmids
- transposons "jumping genes"
- other mobile genetic elements
-- can be freely exchanged bw bacteria
resistance (R) plasmids
- can be transferred to other cells by C, T, and T
- can carry multiple resistance genes
Slideshow 4: Microbial Pathogenesis
paleopathological evidence of a pathogen
Brucellosis in a skeleton from an Australopithecus africanus male predecessor of Homo sapiens
what is a pathogen and what are the 2 types?
- a microbial agent of disease used synonymously with parasite, transmitted directly or indirectly
1. ectoparasite: lives on surface of the host
2. endoparasite: lives inside host's body
what is an infection defined by?
when a pathogen enters or begins to grow on a host
doesn't imply disease until invasiveness or toxicity
Carlos J Finlay and Walter Reed
CJF: first to identify Aedes aegypti as a vector of yellow fever
WR: confirmed this