Ch. 11, 12, 14
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
When naming ethers:
Name the substituents in alphabetical order
What are carbonyl groups?
aldehydes (-CHO) and ketones (C=O)
Where are aldehydes found?
always on the end of the Carbon chain
How to name aldehydes
the e in the alkane name is changed to al
How to name ketones
the e in the alkane name is changed to one
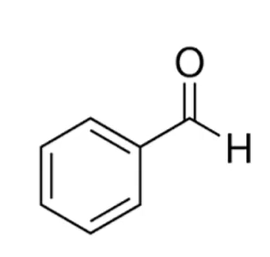
Classify as an aldehyde or ketone.
Give the IUPAC name.
aldehyde; benzaldehyde
Primary alcohols are oxidized to produce
aldehydes (-CHO)
Aldehydes can further oxidize to produce a
carboxylic acid (-COOH)
Secondary alcohols are oxidized to produce
ketones (-C=O)
Do Tertiary alcohols oxidize?
no
Are carboxylic acids polar or nonpolar?
polar
Is a carbonyl (C=O, aldehyde) polar or nonpolar?
polar
Is hydroxyl (-OH) polar or nonpolar?
polar
Rank the functional group from highest polarity & boiling point to lowest:
carboxylic acids
highest
Rank the functional group from highest polarity & boiling point to lowest:
alcohols
middle
Rank the functional group from highest polarity & boiling point to lowest:
ketones/aldehydes
lowest