CHEM 100 - Ch 1: Basics of Chemistry
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
40 Terms
What is the shape/volume of a solid?
definite shape, definite volume

What is the compressibility of atoms in a solid?
slightly compressible, but relatively hard

What is the mobility of atoms in a solid?
relatively stationary and limited movement

What is the spacing of atoms in a solid?
tightly packed and touching, which restricts movement

What is the spacing of atoms in a liquid?
closely packed but not touching, which allows movement

What is the mobility of atoms in a liquid?
flows past each other, which allows movement

What is the compressibility of atoms in a liquid?
slightly compressible, but relatively hard

What is the shape/volume of a liquid?
no fixed shape, definite volume

What is the spacing of atoms in a gas?
very far apart
What is the mobility of atoms in a gas?
moves past each other at high velocities

What is the compressibility of atoms in a gas?
highly compressible

What is the shape/volume of a gas?
no fixed shape, no fixed volume
What is an element?
A pure substance made up of only 1 type of atom

What is a compound?
A pure substance made up of multiple chemically combined elements in its smallest repeatable units
What is a homogeneous mixture?
A mixture with compounds that are uniformly distributed

What is a heterogeneous mixture/solution?
A mixture with compounds that are not uniformly distributed

What are physical properties?
characteristics that are observable by the senses and do not require change in chemical identity
What are chemical properties?
characteristics that describe how the chemical identity changes
What is an intensive property?
a property that does not depend on amount of matter
What is an extensive property?
a property that depends on amount of matter

What is a physical change?
transformation of matter that does not involve a change in chemical composition

What is a chemical change?
transformation of matter that involves a change in chemical composition

What is the multiplier value for Tera (T)?
E12

What is the multiplier value for Giga (G)?
E9

What is the multiplier value for Mega (M)?
E6

What is the multiplier value for Kilo (k)?
E3
What is the multiplier value for deci (d)?
E-1

What is the multiplier value for centi (c)?
E-2

What is the multiplier value for milli (m)?
E-3

What is the multiplier value for micro (μ)?
E-6

What is the multiplier value for nano (n)?
E-9
What is the multiplier value for pico (p)?
E-12
What is accuracy?
how close an experimental value is to a known, accepted value
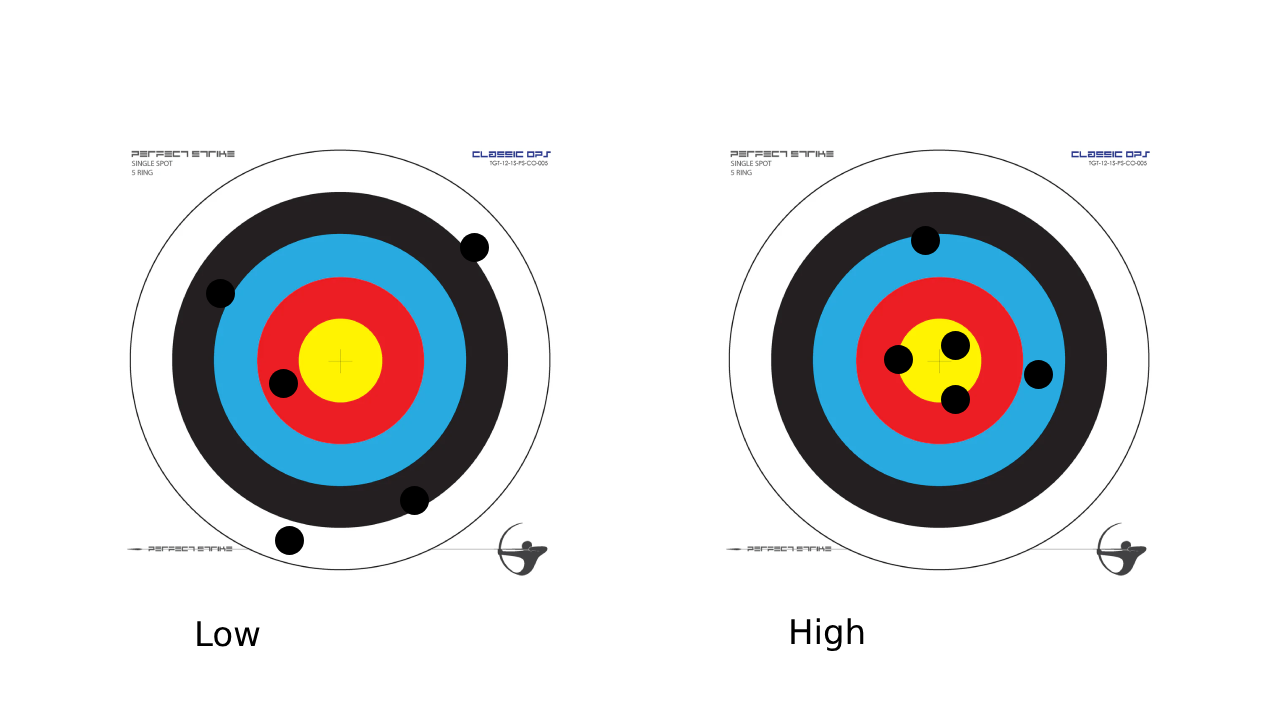
What is precision?
describes reproducibility of the measurements made from an instrument
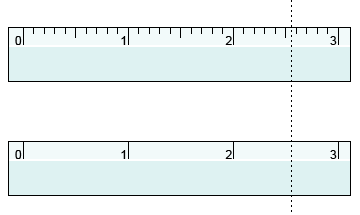
What is the estimated digit?
When using analog measurement instruments, report your measurement one digit past the last digit provided by the instrument
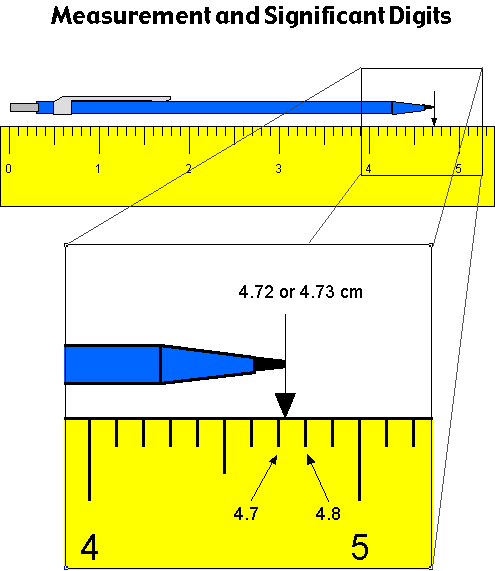
What are exact numbers?
known values that have infinite precision (not considered in determining sig figs)

How many sig figs are there in the final answer of a multiplication or division calculation?
the fewest number of significant digits from the contributing factors

How many sig figs are there in the final answer of an addition or subtraction calculation?
the fewest number of decimal places from the combined numbers

What is a derived unit?
not directly measurable, but must be calculated

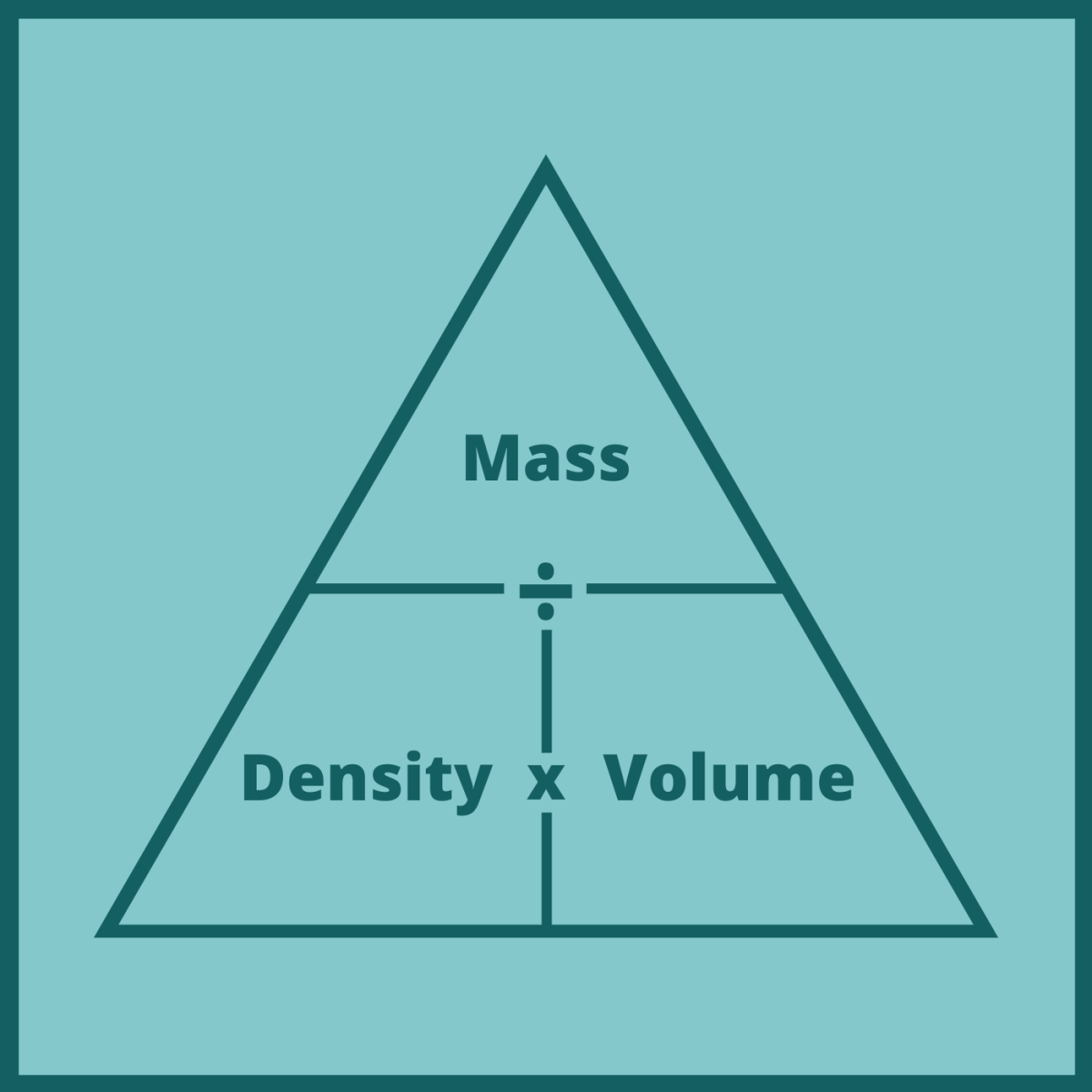
What is the density triangle?
Density = Mass/Volume