chemical energetics
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Formula for enthalpy change
∆H = total energy of bonds broken - total energy of bonds formed
What happens to thermal energy and energy levels during exothermic reactions?
Thermal energy released
Energy level of system decreases, energy level of surroundings increases
Negative enthalpy change
What is the difference between the energy of bond forming and breaking during exothermic reactions?
H(bond forming) > H(bond breaking)
What are some processes that are exothermic reactions?
Neutralisation
Respiration
Inside heat packs and self-heating pots
Why are exothermic reactions more stable than endothermic ones?
Lower final energy level, so lower Ea required
Enthalpy change graph of an exothermic reaction
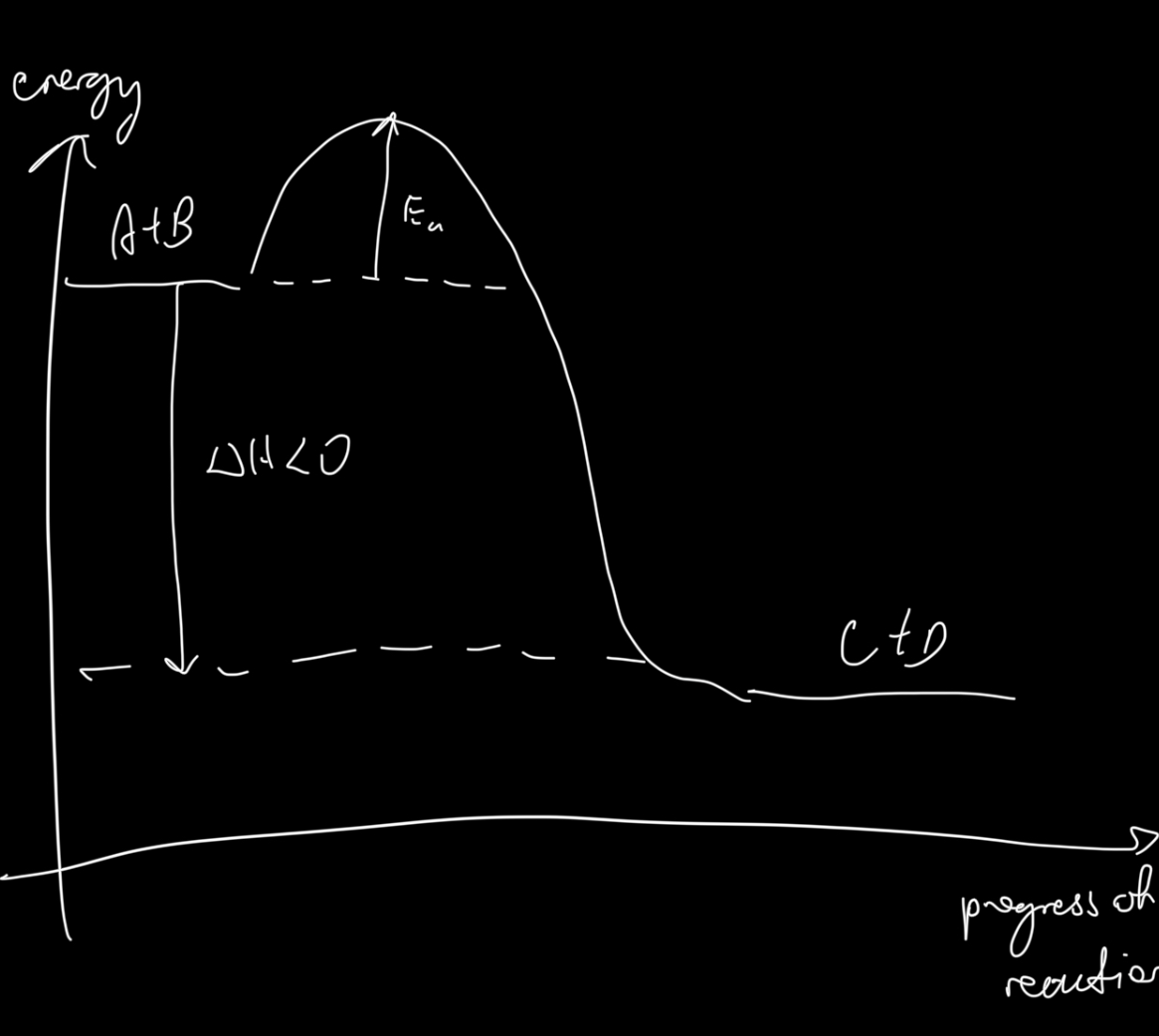
What happens to thermal energy levels during endothermic reactions?
Thermal energy is absorbed
Energy level of system increases, energy level of surroundings decreases
Positive enthalpy change
What is the difference between the energy of bond forming and breaking during endothermic reactions?
H(bond breaking) > H(bond forming)
What are some processes that are endothermic reactions?
thermal decomposition
photosynthesis
inside a cooling pack
Enthalpy change graph of an endothermic reaction
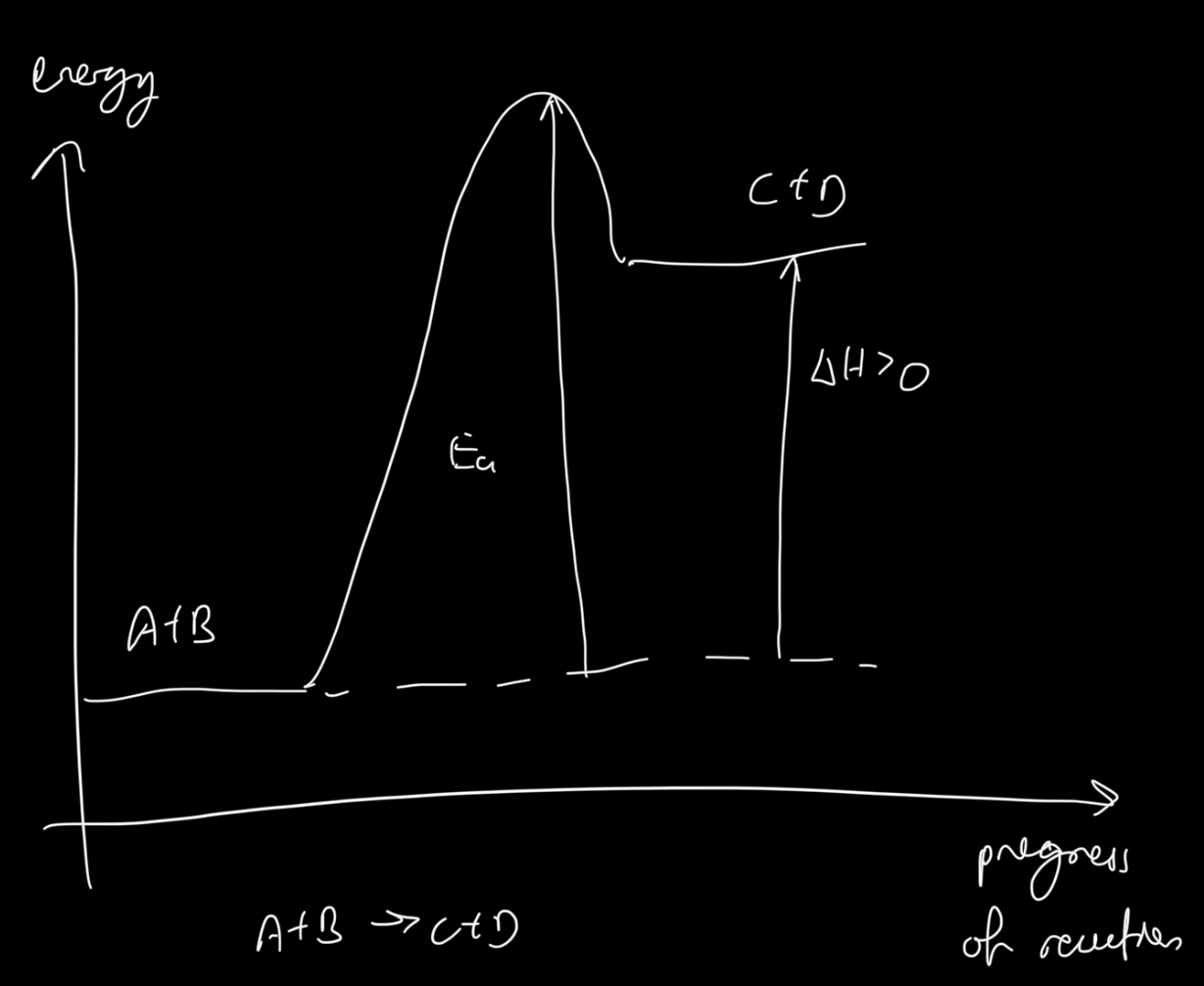
What is bond energy?
The amount of energy absorbed or released to break one mole of a chemical bond
Each chemical bond has a specific bond energy associated with it
What is activation energy? (Ea)
The minimum amount of energy that colliding reactant particles must possess to react with each other