Diamond and Silicon Dioxide
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What state are giant covalent molecules in room temperature?
Solid
Giant covalent molecules have ______ melting and boiling points
high
Why does giant covalent molecules have high melting and boiling point?
Because they have millions of strong covalent bonds (in order to melt or boil these substances, we have to break all of these covalent bonds, which requires a lot of energy)
Diamond is formed by
huge number of carbon atoms joined by covalent bonds
In a diamond, each carbon atom forms
4 strong covalent bonds
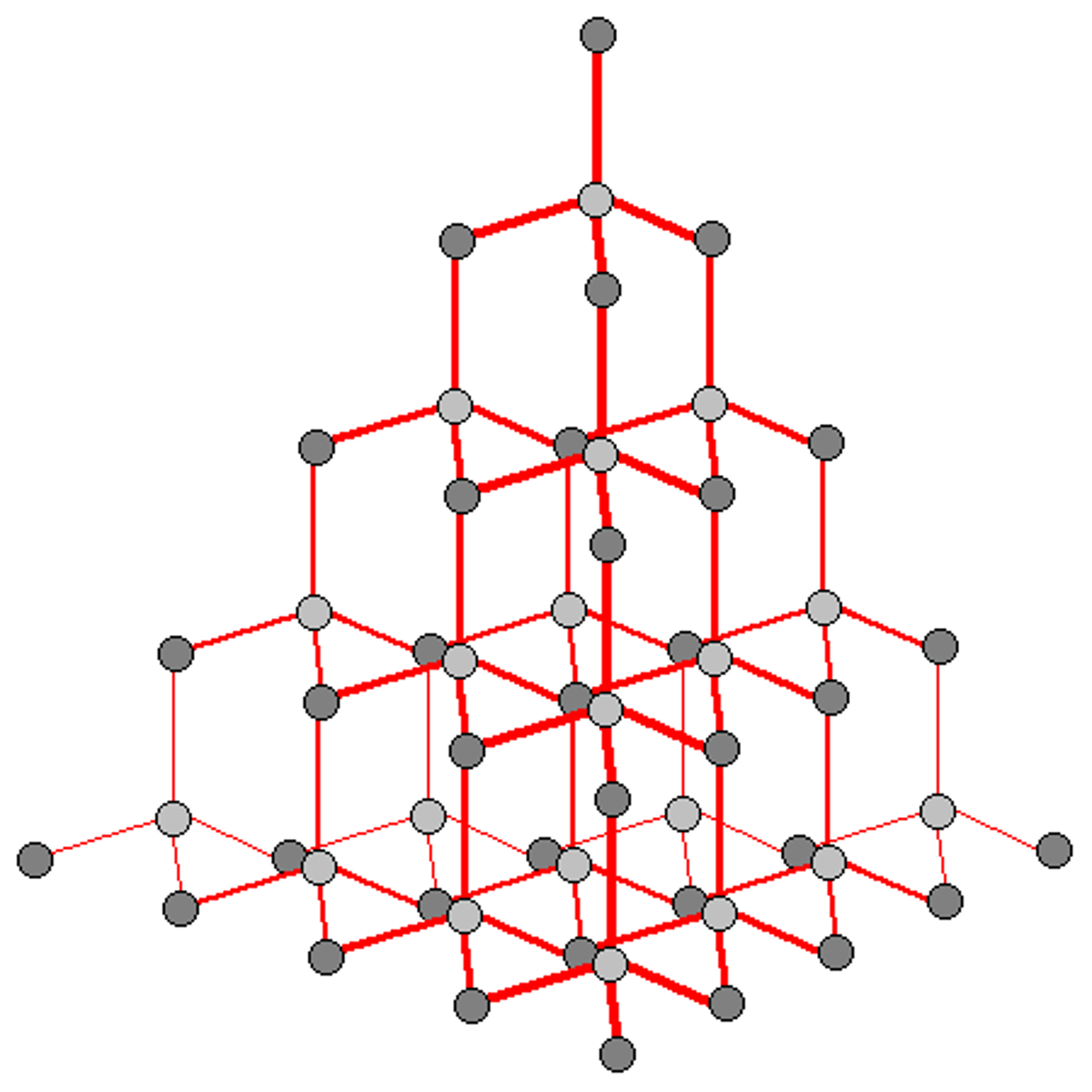
Diamonds requires a great deal of energy to melt because
they have a huge number of covalent bond which needs to be broken, so it has a very high melting/boiling point
Diamond cannot conduct electricity because
all of the outer electrons are in covalent bonds, so there are no free electrons to carry electrical charge
Silicon dioxide contains the elements
silicon and oxygen covalently bonded together
Silicon dioxide have a _______ melting and boiling point
very high
Why does silicon dioxide have a very high melting and boiling point?
a huge number of strong covalent bonds must be broken which takes a lot of energy