(f) acids, alkalis and titrations
1/6
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
using litmus, phenolphthalein and methyl orange between acidic and alkaline solutions (2.28)
litmus colour in acidic solution: red
litmus colour in alkaline solution: blue
phenolphthalein colour in acidic solution: red
phenolphthalein colour in alkaline solution: yellow
methyl orange colour in acidic solution: colourless
methyl orange colour in alkaline solution: pink
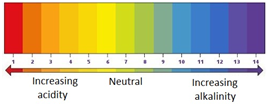
pH scale (2.29)
0-3 → strongly acidic
4-6 → weakly acidic
7 → neutral
8-10 → weakly alkaline
11-14 → strongly alkaline
use universal indicator to measure pH (2.30)
an indicator is a substance that has more than one colour form depending on the pH
an acid is a source of which ions? (2.31)
hydrogen (H+)
an alkali is a source of which ions? (2.31)
hydroxide (OH-)
what do acids and alkalis do to each other (2.32)
neutralise by combining with the hydrogen ions in them
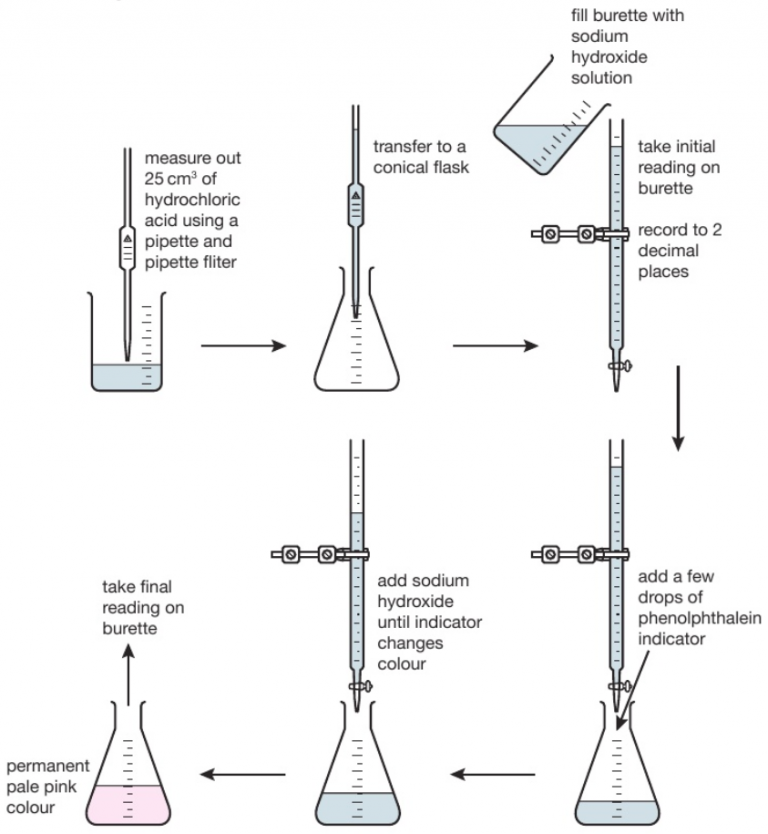
how to carry out an acid-alkali titration (2.33C)
titrations are used to find out precisely how much acid it would take to neutralise a certain volume of alkali or vice versa
the diagram shows the titration method for a neutralisation reaction between hydrochloric acid and sodium hydroxide, using phenolphthalein as an indicator. the indicator changes colour when neutralisation occurs.
the conical flask is swirled to mix the solutions each time alkali is added. when reading the burette it is important to be aware that the numbers on the scale increase from top to bottom. readings are usually recorded to the nearest 0.05cm3 so all readings should be written down with 2 decimal places. the second decimal place is given as a ‘0’ if the level of the solution is on a line or ‘5’ if it is between the lines. the volume of alkali added is calculated by subtracting the final reading from the initial reading. various indicators can be used such as phenolphthalein or methyl orange. however universal indicator should not be used since it has a wide range of colours rather than one specific colour change so it would be unclear when the precise endpoint of titration achieved.
this process is repeated a number of times. the first time it is done roughly to get a good approximation of how much alkali needs to be added. on subsequent attempts, the alkali is added very slowly when approaching the correct volume.