Electronic Structure
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
What do electrons within atoms occupy?
Fixed energy levels or shells.
The number given to each shell is known as what?
The Principal Quantum Number (PQN).
The first shell has a Principal Quantum Number of…
1.
The second shell has a Principal Quantum Number of…
2.
The regions of space around the nucleus where there is a high probability of finding an electron of a given energy is called…
Atomic orbitals.
What are atomic orbitals?
A region in an atom that can hold up to two electrons with opposite spins.
In which direction do the electrons spin?
One electron spins clockwise, whilst the other spins anti-clockwise.
*This is because they repel.
How many electrons can each orbital contain?
2 electrons (maximum).
Orbitals of the same type are grouped together in a…
Sub-shell.
What is a sub-shell?
A division of electron shells seperated by orbitals.
What are the 4 types of atomic orbitals?
s,p,d and f.
What shape does the s orbital have?
Spherical shape.
Every electron shell contains a single…
S orbital.
How many orbitals are there in a s subshell?
1 orbital.
How many electrons can the s subshell hold?
2 electrons.
What shape does the p orbital have?
Dumb-bell shape.
What three dumb-bell shaped orbitals is a p subshell made up of?
Pₓ orbital (horizontal)
Pᵧ orbital (vertical)
Pz orbital (diagonal)
How many orbitals are there in a p subshell?
3 p orbitals.
How many electrons can a p subshell hold?
6 electrons (3 pairs).
How many orbitals are in a d subshell?
5 d orbitals.
How many electrons can a d subshell hold?
10 electrons (5 pairs).
How many orbitals are in a f subshell?
7 f orbitals.
How many electrons can a f subshell hold?
14 electrons (7 pairs).
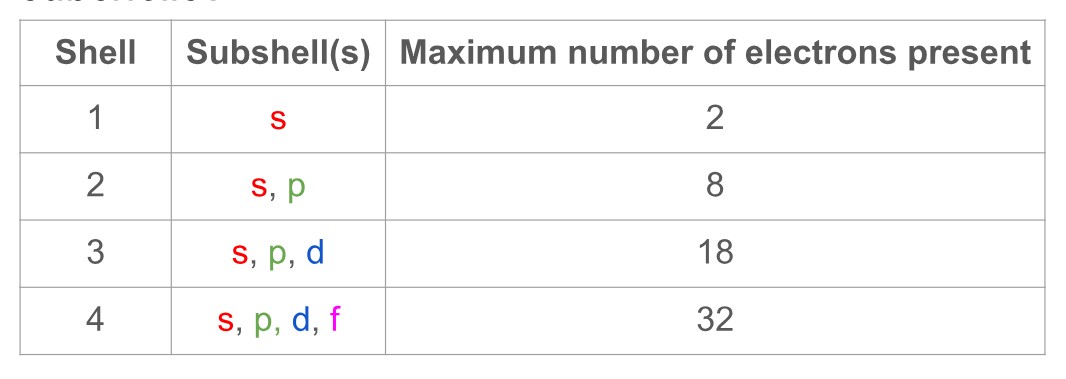
Which electron shells, from 1-4, contain which subshells?
How do electrons fill atomic orbitals?
Electrons fill atomic orbitals in order of increasing energy.
Electrons prefer to occupy orbitals on their own and will only pair up if there is no empty orbital of the same energy available.
What is electronic configuration?
The arrangement of electrons in an atom.
State the 3 basic rules when finding the electronic configuration.
Electrons fill atomic orbitals in order of increasing energy.
A maximum of two electrons can occupy any orbital each with opposite spins.
Each orbital in a subshell will first fill with one electron before pairing starts.
State the order in which atomic orbitals are filled.
1s, 2s, 2p, 3s, 3p, 4s, 3d
Why is the 4s subshell filled before the 3d subshell?
Generally, electrons fill the lowest energy subshell first and only starts a new one when the last subshell is completely filled.
The 4s subshell has a lower energy than the 3d therefore, it is filled first.
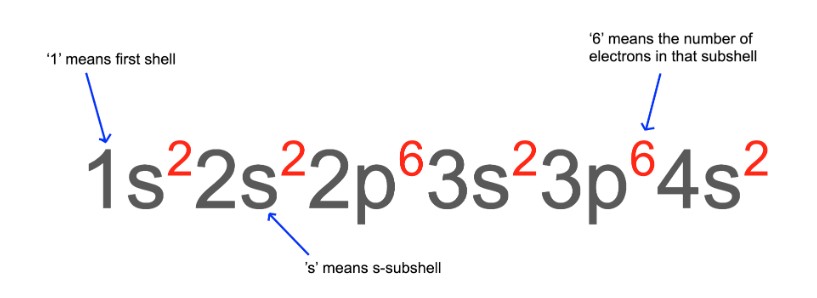
Example: What is the electronic configuration of calcium?
A calcium atom has 20 electrons.
Which 2 elements in the periodic table are exceptions to the rule that the 4s orbital is filled before the 3d orbital?
Chromium (Cr) & Copper (Cu)
The 4s orbital only fills with one electron as this gives them more stable configurations in the 3d orbitals.