Module 6 - Cell Signalling AKA Signal Transduction AKA Cascade Rxn
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
LEC 1
Cell signalling is…
…is the language of cells!
A language that’s critical for everything from development to immunity.
What creature will we discuss to illustrate the basics of cell signaling?
a slime mold called Dictyostelium discoideum
mnemonic: “Dicki the slime mold”
Dictyostelium discoideum starts its life as a collection of single-celled ….
… amoeba. And these amoeba are happily munching on bacteria in the soil, living their best lives.
But when food becomes scarce, these lil creatures don’t just give up. They band together!
What do Dictyostelium discoideum do when they run out of food?
They aggregate and form a multicellular slug! Like the slimy things you find in your garden.
This IS a slime mold slug.
What is a slime mold?
A "slime mold slug" refers to a temporary, multicellular stage in the life cycle of certain types of slime molds (called cellular slime molds) where individual amoeba-like cells aggregate together to form a slug-shaped mass that can move around
Is a slime mold slug a true slug?
no! this "slug" is not a true slug, but rather a collection of cells behaving as one unit.
A slime mold slug will …. in response to running out of food.
This is so that ….
A slime mold will agreggate tg to form a multicellular slug in response to running out of food.
Moving towards more favorable conditions is a survival strategy.
So Dictyostelium discoideum will become a slime mold slug which will become a…
…a fruiting body!
Dicki —> slime mold —> fruiting body
What is a fruiting body?
fruiting body = the spore-producing organ of a fungus,
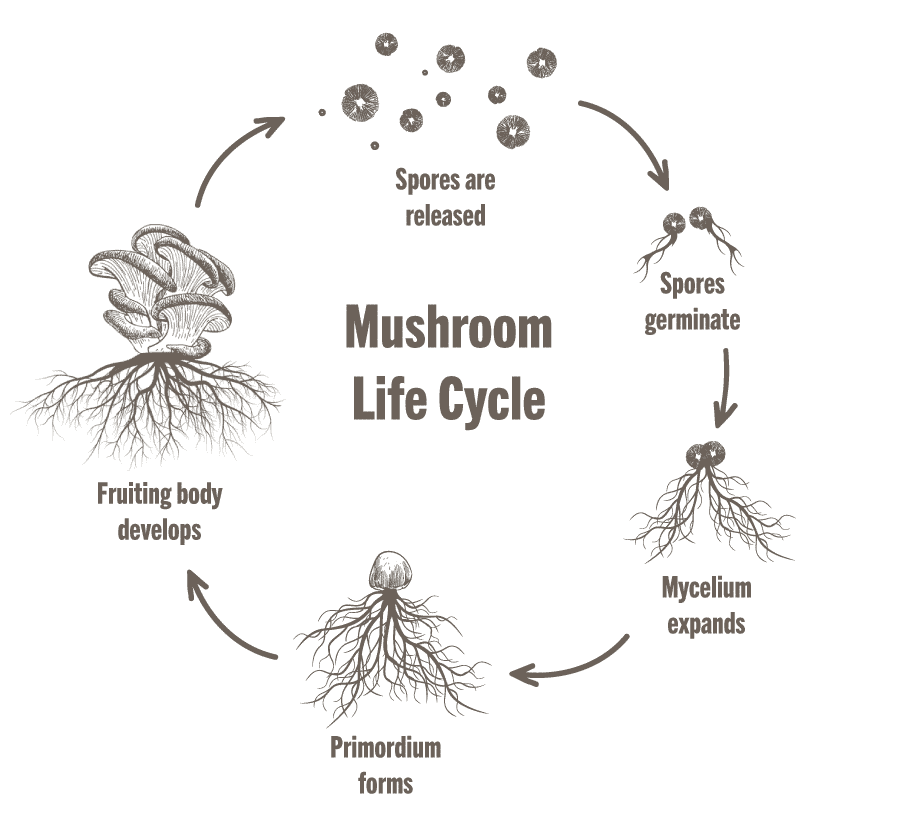
Why does Dictyostelium discoideum turn into a fruiting body?
fruiting bodies release spores that can survive until conditions improve!
The whole process of individual ameoba to a coordinated slug is orchestrated by…
…by cell signalling!
What is the special signalling molecule that Dictyostelium discoideum use?
cAMP = Cyclic adenosine monophosphate
When Dictyostelium discoideum start running out of food, they release cAMP. How do they actually respond to the cAMP signal?
via a receptor of course! a transmembrane protein called GPCR (GP protein coupled receptor).
GCPRs sit on the surface of amoeba acting like cellular antennae. they’re specifically designed to bind certain molecules. in thsi case, the GCPR is tuned to pick up the cAMP signal.
Once cAMP binds to GCPR, it sets off a chain rxn (cascade signalling) inside the cell.
cytoskeleton reorganizes to allow amoeba to move towards the source of cAMP.
and rem. from last lec. that the clatherin coated vesicles is responsible for transporting that GPCR to the cell surface.
Some of a cell’s internal skeleton (cytoskeleton) is made up of a protein called a[. ].
ACTIN!
rem. microbio!!
microfilaments are made up of actin, while microtubules are made up of tubulin.
So if clatherine is mutated, what consequence will follow?
then the GPCR can’t get where it needs to be to receive cAMP.
so the cell will basically be deaf to the signal.
This signalling mechanism is also critical in our human bodies, in __________ cells!
immune cells!
Neutrophils ( white blood cells) are always ready to fight. But how do they know where to go to fight off infections? cuz of a peptide signal called FMLP (Formulated Methionine, leucine, phenylalanine). This FMLP is a breadcrumb trail that neutrophils can follow. Neutrfils have a GPCR on their surface that recognizes this FMLP peptide.
SIGNALLING =
SIGNALLING = cells talking to one another to INDUCE an action. this signal leads to a change in the behavior of the receiving cell.
Summarize the steps of cellular signalling:
signalling cell has to produce and release signal molecule (e.g. cAMP)
receiving cell has to have a receptor that can bind the signal (e.g. GPCR)
signal must iterate inside the target cell (cascade rxn)
target cell undergoes a change in behavior
Fast vs slow cellular responses:
fast usually involves activating enzymes that are already present within the cell.
slow requires building those new proteins from scratch.
How do scientists measure signalling?
-assess receptor’s affinity for cell
-determine concentration of signal needed to trigger a physiological response
Remember what does a low dissociation constant (KD) or higher eqbm constant (Keq) mean?
low KD = high Keq = high affinity!
What are the TYPES of signalling? recall 4T03
endocrine = over long distances (cells release hormones into bloodstream)
paracrine = to the next neighbor (cells release signals that act locally affecting nearby cells)
proximal = cells in direct contact w/ each other
autocrine = cell signals to ITSELF
What do endocrine signals regulate?
sleep cycle, metabolism
What is an example of paracrine signals?
growth factors, neurotransmitters (relay signals btwn neurons),
In proximal signalling, the signal and receptor can actually be __________________ ____________ on the surface of those two cells. e.g. gap junctions
transmembrane proteins
What do autocrine signals regulate?
cell growth, division
Cells get bombarded with a bunch of signals from their environment.But how do they know which signals to prioritize, integrate, and make decisions that translate into action?
well scientists are tryna figure it out!
Cancer is often characterized by uncontrolled cell growth. Which can be caused by mutations in genes that regulate cell signalling.
So a glitch in the comms system can lead to a cell going rogue and dividing outta control!
LEC 2
We will learn about two cell signalling pathways in this lecture. What are they?
-making RBCs
- cell differentiation,
cell survival or apoptosis, cell division and
proliferation, or changes in cell metabolism
How the body knows how to make more RBCs: What signal sets it off?
erythropoietin (EPO)!
Where does erythropoietin come from and what does it do?
erythropoietin mainly comes from the kidneys and it travels thru the bloodstream to tell target cells to make more RBCs.
Since erythropoietin travels thru the bloodstream, what kind of signalling is it?
endocrine!
Which cells does erythropoietin target?
the cells that have an EPOReceptor! EPOR is the door that only opens for the key EPO.
Since erythropoietin is a cytokine, then the erythropoietin
receptor is…
.. a cytokine receptor.
Once EPO finds its matching EOR, what next?
EPO binds to EPOR to form a dimer (a quaternary protein composed of two proteins (subunits)),
Each EPOR has an enzyme attached to it called a JAK-kinase. All these JAK-kinases are ___________ until the receptors come together (dimerization).
Each EPOR has an enzyme attached to it called a JAK-kinase. All these JAK-kinases are inactive until the receptors come together (dimerization).
Why do the JAK-kinases only activate once the receptors dimerize?
cuz it’s what brings them together!
Once JAK-kinases get activated, they’re called ____________ kinases cuz they add phosphate groups to tyrosine residues on proteins.
inactive JAK-kinases —> activate —→ tyrosine kinases
Now the signal has to travel further into the cell. The phosphorylated tyrosines then become docking sites for other proteins. They send out a signal called:
STAT 5!
STAT 5 has a special domain called SH2. SH2 is perfectly shaped to fit into those…
…those phosphorylated tyrosines.
So STAT 5 binds to the phosphotyrosine —>brings it close to the activated JAK-kinase which is still on the receptor —> then JAK-phosphorylates …
…JAK phospholates stat 5!
Phosphorylated STAT 5 pairs up with another phosphorylated STAT 5 (dimerization). This dimer is the key to getting the signal into the nucleus. Why the nucleus?
cuz that’s where the DNA at!! STAT 5 is a transcription factor so it can bind to DNA, turn on specific genes, to tell it to make more RBCs.
STAT 5 is a ______________ factor.
transcription factor!
What about when the signal needs to be turned off?
we can't have red blood cell production going on forever. too many red blood cells can actually lead to strokes or heart attacks.
a phosphotase enzyme called SHP1 removes phosphate from the JAK-kinase to deactivate them
Recall that for phosphorylation, ____________ add phosphate groups.
For de-phosphorylation, _________________ remove phosphate groups.
Recall that for phosphorylation, kinases add phosphate groups to activate proteins.
For de-phosphorylation, phosphotases remove phosphate groups to deactivate proteins.
What is a more extreme solution to stopping RBC production than the phosphotase SHP1?
a protein called SOCS. it can block stat proteins from binding to the receptor in the first place. or it can tag the JAK-kinases for destruction (rem. ubiquitination).
The JAK-STAT pathway is shorter in people with full length EPO receptors because they’re highly sensitive to those negative regulators like SHP1 and SCOS.
So the JAK-STAT pathway is longer in people with truncated EPO receptors cuz they’re less sensitive to “ . —>
—> so they have HIGHER RBC production! like athletes who dope.
Why would an athlete dope?
Well, in this case, the increased red blood cell count
leads to increases in the capacity of the athlete to
carry oxygen and increases endurance. Especially
during aerobic activities. Y e t while this may seem
like a reasonable approach to take, this is actually a
very dangerous practice that can be quite lethal
Now we will discuss the second pathway…
…
RTK =
Receptor Tyrosine Kinases (or RTKs) which are associated with Ras G-protein activation
Just like the cytosine receptors, RTKs also dimerize when they bind to their ligands. But instead of activating JK, dimerization activates a pathway involving…
…involving ROS! our famous G-protein.
ROS is like a switch. When it’s bound to GTP, it’s on. When it’s bound to GDP, it’s off.
And…
And ROS can actually break down GTP into GDP + Pi. i.e. it can turn itself off.
this is thru GTPase activity
What other G-proteins work with ROS?
GFS, GGPS, GDIS
GNEF = guanine nucleotide exchange factor = makes it easier for ROS to swap out GDP for GTP = helps it turn on
GAP = GTP’s activating protein to incite GTPase activity = turns it off faster
GNDI = guanine nucleotide dissociation inhibiter = keeps ROS firmly bound to GDP to prevent it from accidentally turning on by GNEFs = safety lock
But how does the signal get from RTK to ROS in the first place?
an adapter protein called GRB2 has an SH2 domain AND an SH3 domain to connect proteins.
SH2 domain binds to a specific phosphorylated tyrosine on the activated RTK.
SH3 domain binds to proline rich region on SOS.
What is the SOS protein?
it is the GNEF for ROS.
Activating ROS triggers a whole chain rxn called a ___________ cascade because….
Activating ROS triggers a whole chain rxn called a kinase cascade because each one activates the next, like dominoes falling over.
Show me the kinase cascade, where each arrow represents an activation.
ROS —> RAFMAP ——> MEK —→ ERK12
T/F: The MAP kinase is the final messenger in the cascade.
true!
Where does the MAP kinase go?
to the nucleus!
Inside the nucleus, it has a few targets.
One of them is another kinase called P90 RSK. MAP phosphorylates P90 which allows P90 to move to the nucleus to.
MAP kinase phosphorylates a transcription factor called TCF.
While P90 phosphorylates another transcription factor called SRF.
What do they do?
TCF and SRF work together by binding a specific DNA sequence called the serum response element (or SR).
What is SR?
SR is an enhancer. it increases the likelihood that a nearby gene will be transcribed. so when TCF and SRF bind to SR ( a volume knob), they basically crank up the volume on nearby genes.
—> promoting their expression.
What are the key genes being regulated by SR?
-COS. which encodes another transcription factor which regulates the cell cycle (growth and division)
Cancer is often a consequence of disrupted signalling pathways. When the signals for growth and division are always on…
…well cells will just proliferate unchecked and that results in tumor formation!
LEC 3
What does GPCR stand for?
GPCR = G-protein coupled receptor
GPCRs are super common receptors all over the human body and are involved in pretty much EVERY physiological process you can think of.
What is the topology of a GPCR?
it’s serpentine! so it weaves in and out of the membrane 7 times.
-the outer proteins act like docking stations for signals coming into the cell (like hormones or neurotransmitters)
-the internal proteins are connection pts to G-proteins inside the cell which transduce the signal into the cell
When we get stressed, our bodies release catacolamines like epinephrine and norepinephrine which travel thru the bloodstream until they reach their destination: ______________.
GPCReceptors!
There are different kinds of adinuric receptors that epinephrine can bind to:
alpha 2 receptors and beta receptors
Alpha receptors tend to be [inhibitory/stimulatory] and beta receptors tend to be [inhibitory/stimulatory].
Alpha receptors tend to be inhibitory and beta receptors tend to be stimulatory.
When epinephrine binds to beta receptors in liver and fat cells, it tells the cells to…
…to break down stored energy! by giving a quick boost of glucose and fats.
When epinephrine binds to beta receptors in heart muscle cells, it tells the cells to…
….make the heart beat faster and stronger!
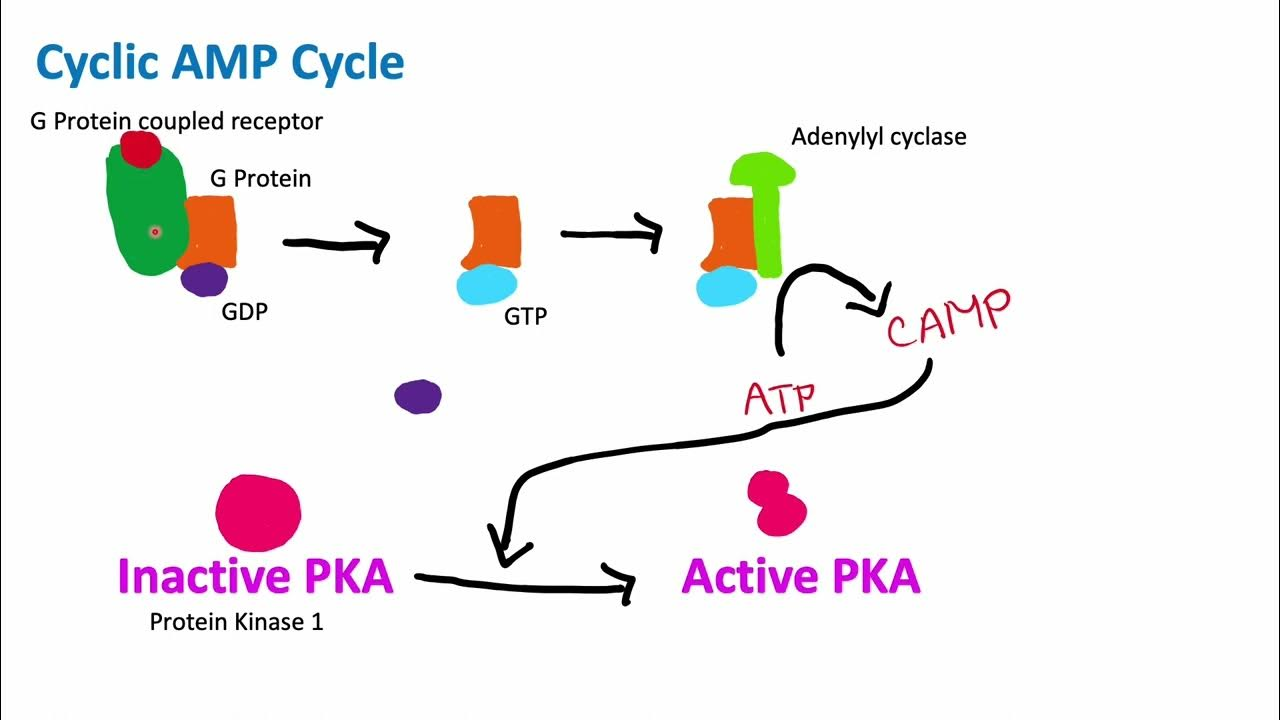
Once epinephrine binds to the GPCR, a conformational change happens that allows it to interact with the G protein.
Then:
then the activated GPCR nudges the G protein and causes it to release GDP and grab onto GTP instead. Where does the G protein go?
The G protein that is holding onto the GTP in its alpha subunit goes to adenine cyclists. What does adenine cyclist do?
adenine cyclist converts ATP into cyclic AMP (cAMP).
cAMP passes the baton onto…
… protein kinase A (pKA).
Inactive pKa is a structure of..
….of 2 regulatory subunits + 2 catalytic subunits
Activated pKa do what?
Activated pKa phosphorylate other proteins!
In muscle cells, pKa tells them to..
to break down glycogen! fast energy in da muscle
In liver cells, pKa tells them to
to break down glycogen again, releasing glucose into the bloodstream for other tissues. But it also stops the liver from making more glycogen.
Focus on mobilizing energy, not storing it.
pKa can also go into the nucleus, to phosphorylate CRE sequences. which control glucose expression.