Chapter 3: Biological Macromolecules Structure and Function
1/145
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
146 Terms
Biological Macromolecules
Four major classes of macromolecules: Carbohydrates, Lipids, Proteins, Nucleic Acids.
Organic molecules
All contain carbon and may also contain hydrogen, oxygen, nitrogen, and some other elements.
Macromolecule synthesis
The process of linking monomers together to form polymers.
Dehydration synthesis
A reaction where two molecules are linked together by a covalent bond, forming a dimer and releasing a water molecule.
Hydrolysis
The process of breaking polymers down into individual monomers, using water as a reactant.
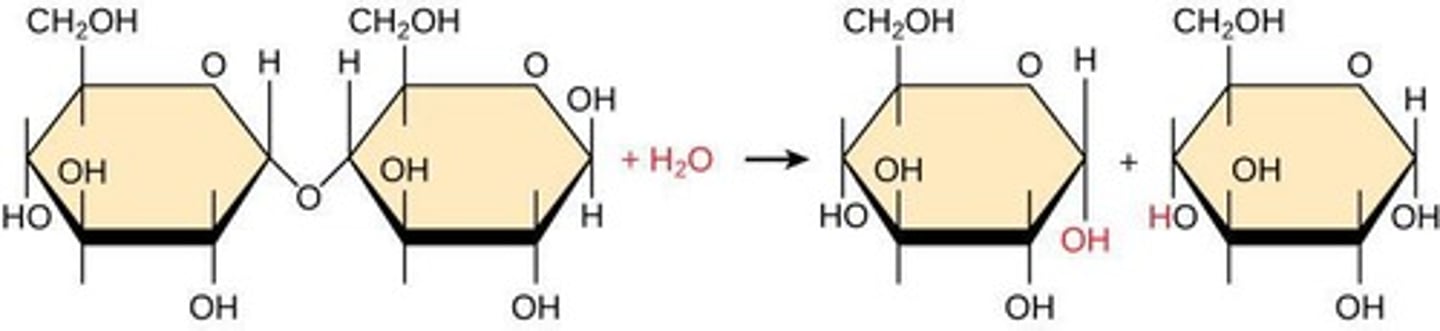
Disaccharide maltose
Formed by linking two glucose molecules through dehydration synthesis.
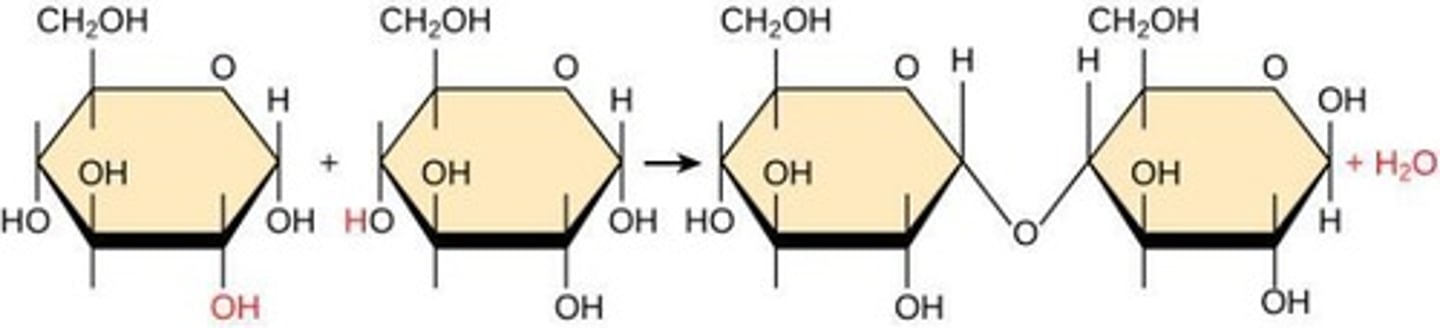
Enzymes
Biological molecules that catalyze or speed up reactions, including hydrolysis and dehydration reactions.
Amylase
An enzyme that breaks down carbohydrates.
Lipases
Enzymes that break down lipids.
Pepsin
An enzyme that breaks down proteins.
Carbohydrates
Organic compounds found in grains, fruits, and vegetables that provide energy to the body in the form of glucose.
General formula of carbohydrates
Represented by the formula (CH2O)n.
Carbohydrate ratio
The ratio of Carbon:Hydrogen:Oxygen in carbohydrates is 1:2:1.
Monosaccharides
Simple sugars usually having 3-7 carbons, bound to a hydroxyl group, and ending with the suffix -ose.
Aldoses
Monosaccharides with a carbonyl group at the end of the carbon chain.
Ketoses
Monosaccharides with a carbonyl group in the middle of the carbon chain.
Trioses
Monosaccharides that contain three carbons.
Pentoses
Monosaccharides that contain five carbons.
Hexoses
Monosaccharides that contain six carbons.
Glucose
An important source of energy and a hexose monosaccharide.
Galactose
A hexose monosaccharide that is part of lactose, also known as milk sugar.
Fructose
part of sucrose / fruit
Monosaccharides
exist as linear chain or ring-shaped molecules
Ring structure of monosaccharides
assumed in aqueous solution
Five- and six-carbon monosaccharides
exist in equilibrium between linear and ring forms
Glycosidic bond
formed when two sugar monomers are joined by a dehydration reaction
Disaccharide formation
occurs when two monosaccharides are linked in a dehydration reaction
Example of disaccharide formation
Glucose + Fructose = Sucrose (disaccharide)
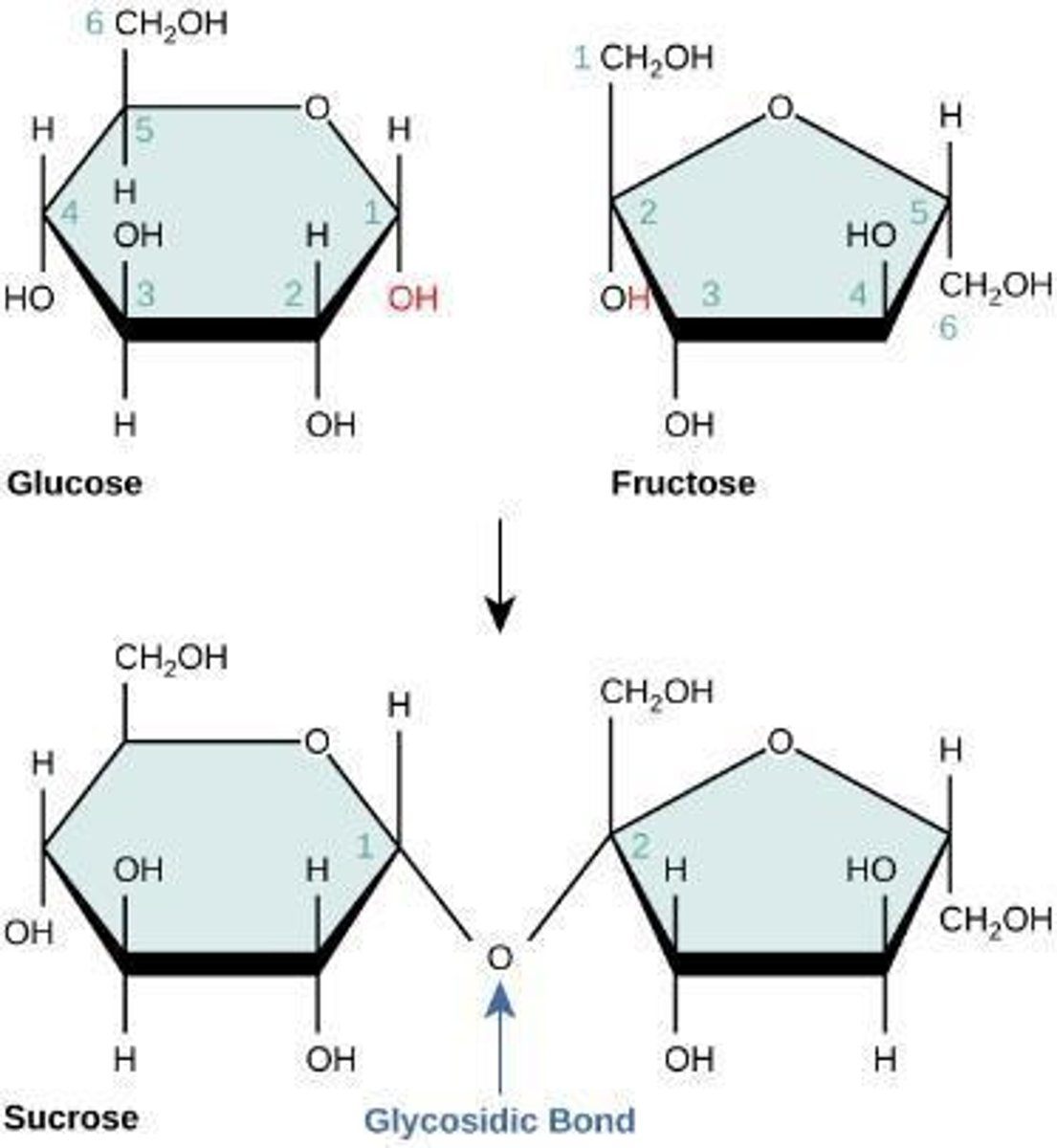
Glycosidic linkage
formed between carbon 1 in glucose and carbon 2 in fructose
1,2 glycosidic linkage
results from the glycosidic bond between glucose and fructose
Common disaccharides
maltose (grain sugar), lactose (milk sugar), sucrose (table sugar)
Polysaccharide
long chain of monosaccharides joined by glycosidic linkages
Polysaccharides
may be branched or unbranched and consist of multiple types of monosaccharides
Molecular mass of polysaccharides
could be > 10,000 amu
Starch
energy storage in plants
Cellulose
cell walls of plants
Chitin
cell walls of fungi and exoskeleton of arthropods
Glycogen
energy storage in animals
Amylose
unbranched glucose monomers in α 1-4 glycosidic bonds
Amylopectin
branched glucose monomers in α 1-4 and α 1-6 glycosidic bonds
Cellulose structure
glucose monomers linked in unbranched chains by β 1-4 glycosidic linkages
Chitin
the hard exoskeleton of arthropods and cell walls of fungi
Lipids
a diverse group of non-polar hydrocarbons
Functions of lipids
long-term energy stores, insulation, building blocks for hormones, important component of cellular membranes
Types of lipids
Fats & Oils, Waxes, Phospholipids, Steroids
Fats
contain two main components: glycerol and fatty acids
Triacylglycerol
formed by joining three fatty acids to a glycerol backbone
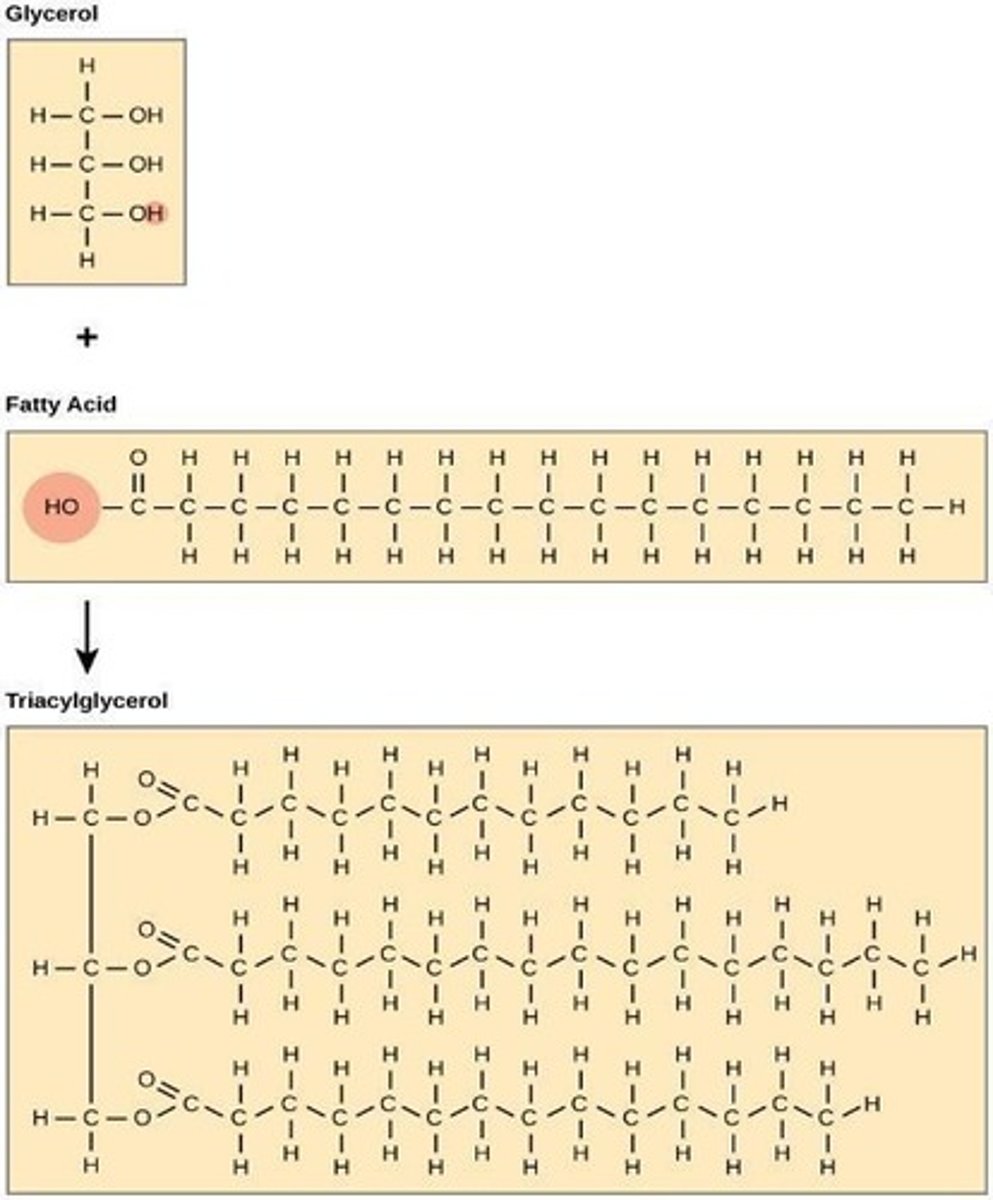
Ester linkage
the bonds formed between glycerol and fatty acids
Saturated fatty acids
Contain no carbon-carbon double bonds.
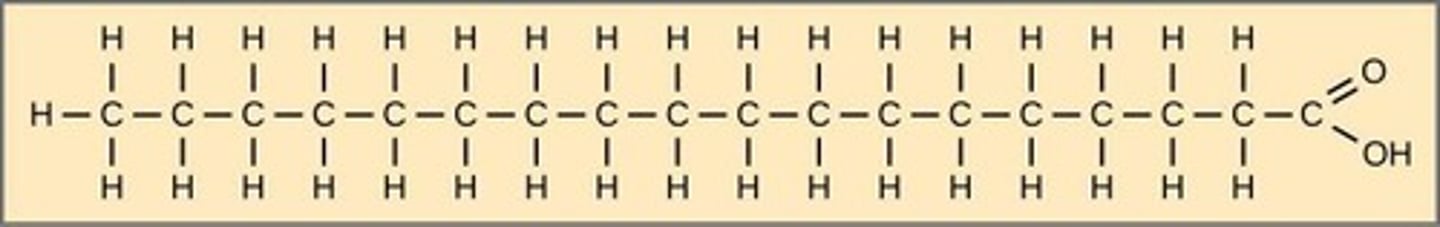
Saturated fatty acids
Pack tightly and are solid at room temperature (butter, fat in meats, etc.).
Saturated fatty acids
May be associated with cardiovascular disease - should be limited in your diet.
Unsaturated fatty acids
Contains at least one carbon-carbon double bond.
Monounsaturated fat
One double bond.
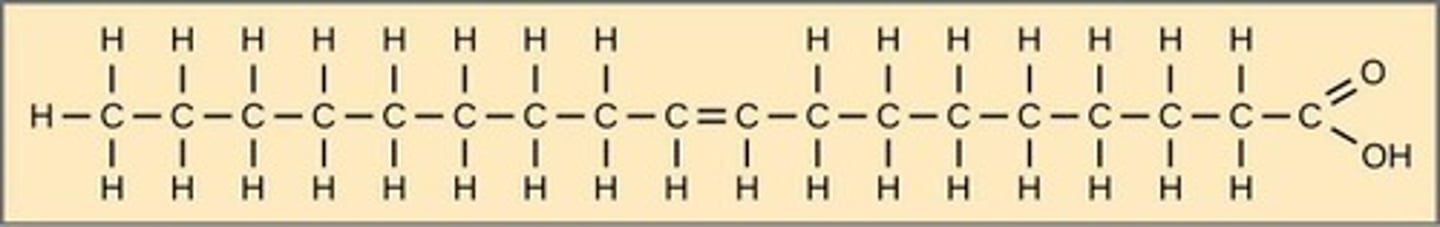
Polyunsaturated fat
More than one double bond.
Unsaturated fats
Most are liquids at room temperature - referred to as oils.
Cis configuration
Hydrogens on the same side of the chain.
Trans configuration
Hydrogens on opposite sides of the chain.
Cis-acids
Have a kink in the chain and cannot be packed tightly.
Cis-acids
Liquid at room temperature.
Trans-acids
Have no kink and can be created through processing.
Trans fats
Foods with trans fat may increase LDL cholesterol in humans (bad for heart).
Essential fatty acids
Required but not synthesized by the body - must be part of diet.
Alpha-linolenic acid
An example of an omega-3 fatty acid.
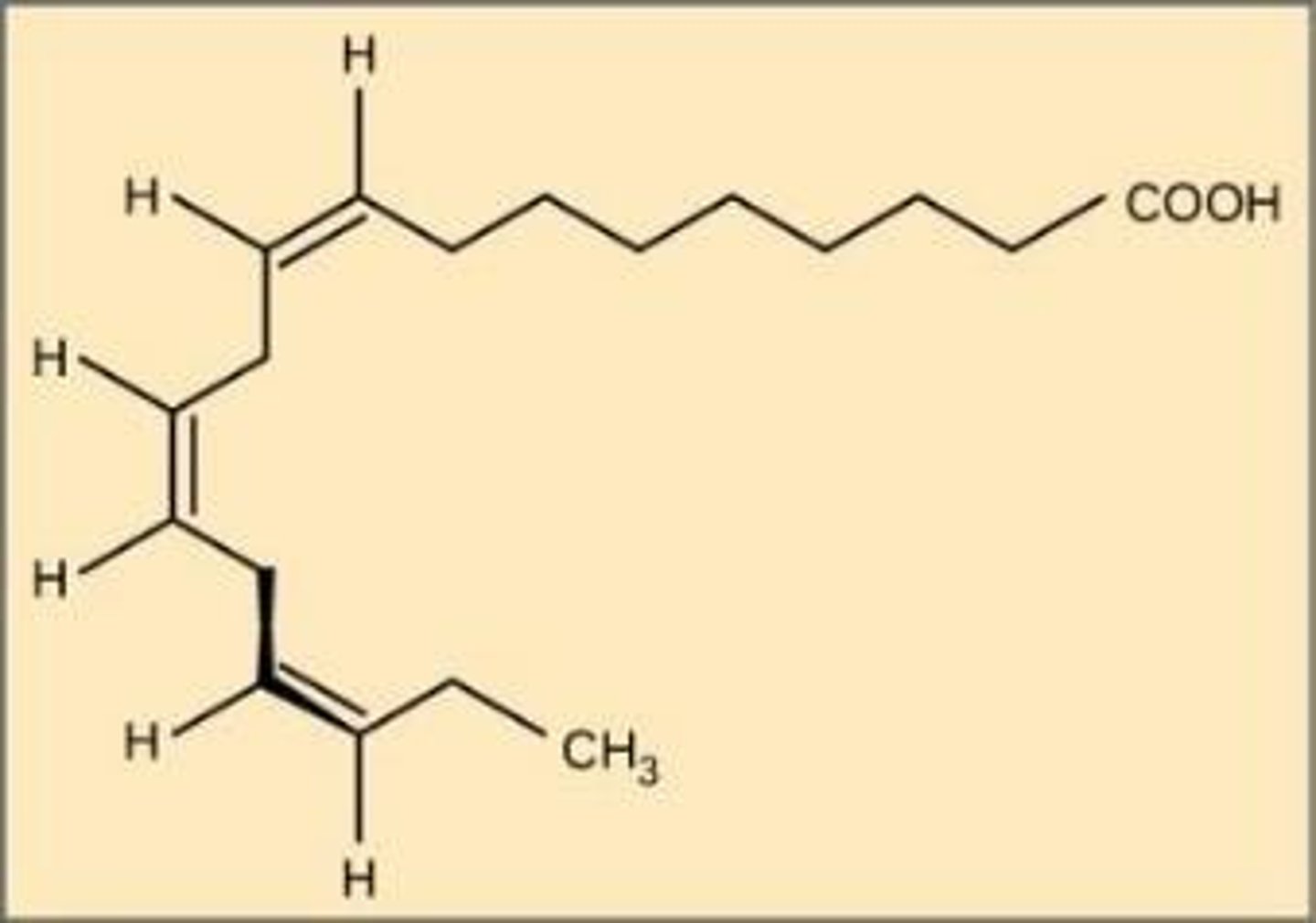
Omega-3 fatty acids
Found in salmon, trout, tuna; these fats are heart healthy.
Omega-3 fatty acids
Reduce risk of heart attack, reduce triglycerides in blood, lower blood pressure.
Waxes
Hydrophobic and prevent water from sticking to surface.
Waxes
Found on the feathers of some aquatic birds and on the surface of leaves from certain plants.
Phospholipid
Molecule with two fatty acids and a modified phosphate group attached to a glycerol backbone.
Phospholipids
Are amphipathic, having a hydrophobic portion and a hydrophilic portion.
Phospholipids
Major constituents of the plasma membrane.
Amphipathic molecule
The hydrophilic heads of the phospholipids face the aqueous solution while the hydrophobic tails are sequestered in the middle of the bilayer.
Steroids
Have a closed ring structure with four linked carbon rings.
Steroids
Many have a short tail.
Steroids
Are hydrophobic.
Cholesterol
The most common steroid, synthesized in the liver, and a precursor to hormones such as testosterone and estradiol, as well as vitamin D.
Lipids
Four major types of lipids that play roles in energy storage, cell structure, and signaling.
Saturated fatty acids
Fatty acids with no double bonds between carbon atoms.
Unsaturated fatty acids
Fatty acids that contain one or more double bonds between carbon atoms.
Phospholipids
Molecules that make up cell membranes, consisting of two fatty acids, a glycerol unit, and a phosphate group.
Proteins
The most abundant organic molecules in organisms, performing a diverse range of functions including regulatory, structural, protective, transport, and catalytic roles.
Enzymes
Catalysts in biochemical reactions, most of which are proteins, with names typically ending in -ase.
Catabolic enzymes
Enzymes that break down substrates.
Anabolic enzymes
Enzymes that build more complex molecules.
Digestive enzymes
Enzymes such as amylase, lipase, pepsin, and trypsin that help in the digestion of food by catabolizing nutrients into monomeric units.
Transport proteins
Proteins like hemoglobin and albumin that carry substances in the blood or lymph throughout the body.
Structural proteins
Proteins such as actin, tubulin, and keratin that construct different structures, like the cytoskeleton.
Hormones
Proteins like insulin and thyroxine that coordinate the activity of different body systems.
Defense proteins
Immunoglobulins that protect the body from foreign pathogens.
Contractile proteins
Proteins such as actin and myosin that are involved in muscle contraction.
Storage proteins
Proteins that provide nourishment in early development of the embryo and the seedling, such as legume storage proteins and egg white (albumin).
Amino acids
The monomers that make up proteins, consisting of a central carbon atom, an amino group, a carboxyl group, a hydrogen, and a side chain (R-group).
Essential amino acids
Amino acids that must be supplied in the diet for humans, including isoleucine, leucine, and cysteine.
Peptide bonds
Links formed between amino acid monomers through dehydration synthesis reactions, where the carboxyl group of one amino acid is linked to the amino group of another.
Polypeptide
A chain of amino acids joined together in peptide linkages.
Protein
A polypeptide or multiple polypeptides with a biological function, often combined with non-peptide groups.
Primary structure
The first level of protein structure, determined by the sequence of amino acids.
Secondary structure
The second level of protein structure, involving the folding or coiling of the polypeptide chain.
Tertiary structure
The third level of protein structure, the overall three-dimensional shape of a single polypeptide.
Primary structure
the unique sequence of amino acids in a polypeptide
Sickle cell anemia
a condition where a change in one amino acid (glutamic acid replaced by valine) can impact human health