The reactivity series/Ionic equations
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
What happens when a metal reacts with water
Metal hydroxide + Water
They also lose electrons to form positive ions
Which form meats react with water
Potassium,sodium,lithium and calcium
How is a metals reactivity determined
How easily they lose electrons-forming positive ions
Name the metals in order of reactivity series
Potassium
Sodium
Lithium
Calcium
Magnetism
Carbon
Zinc
Iron
Hydrogen
Copper
Ions that you should remember
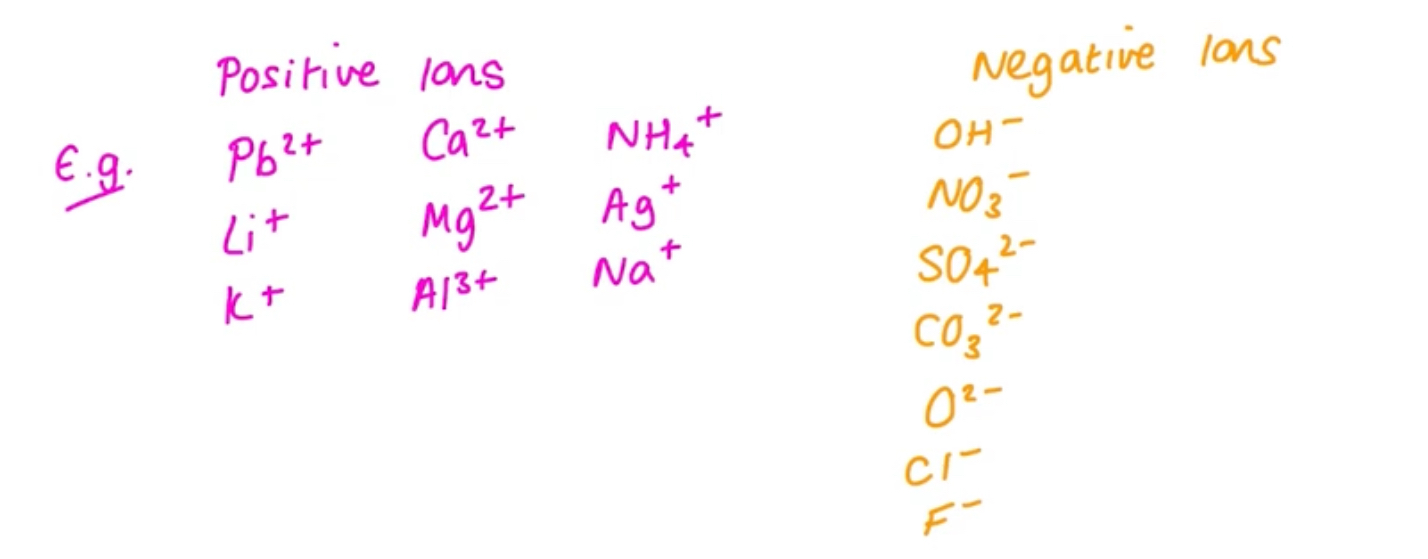
What ionic charge does zinc(Zn) form
2+
What ionic charge does silver(Ag) from
+1
What charge is sulfate
SO42-
What charge is carbonate
CO32-
What charge is nitrate
NO3-
Mg»»Mg2+ 2e-
Is this reduction or oxidation
This is oxidation as we see Mg is at 0 but then goes to 2+ at Mg2+ showing it gained oxygen . So this is a oxidation
What is the equation for Sulfuric acid
H2SO4
What is the equation for Nitric Acid
HNO3
What do acids produce in aqueous solutions
H+ ions
Write the equation when Sulfuric acid is dissolved in water
H2SO42→»2H+ + 2SO42-
What is a base
A base is a chemical that can neutralise acids to form salt and water
What are the two types of bases
Metal oxides or metal hydroxides
What is a alkali
A base that dissolves in water to form OH- ions(hydroxide)
How can we determine the Ph of a solution
Ph probe-Meaures Ph electronically by given a numerical value and is more accurate
Universal indicator- changes colour depending on certain Ph
What is the neutralisation reaction
Acid + base» Salt + water
H+ + OH» H2O