Lecture 17: Immunity at Mucosal Surfaces
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
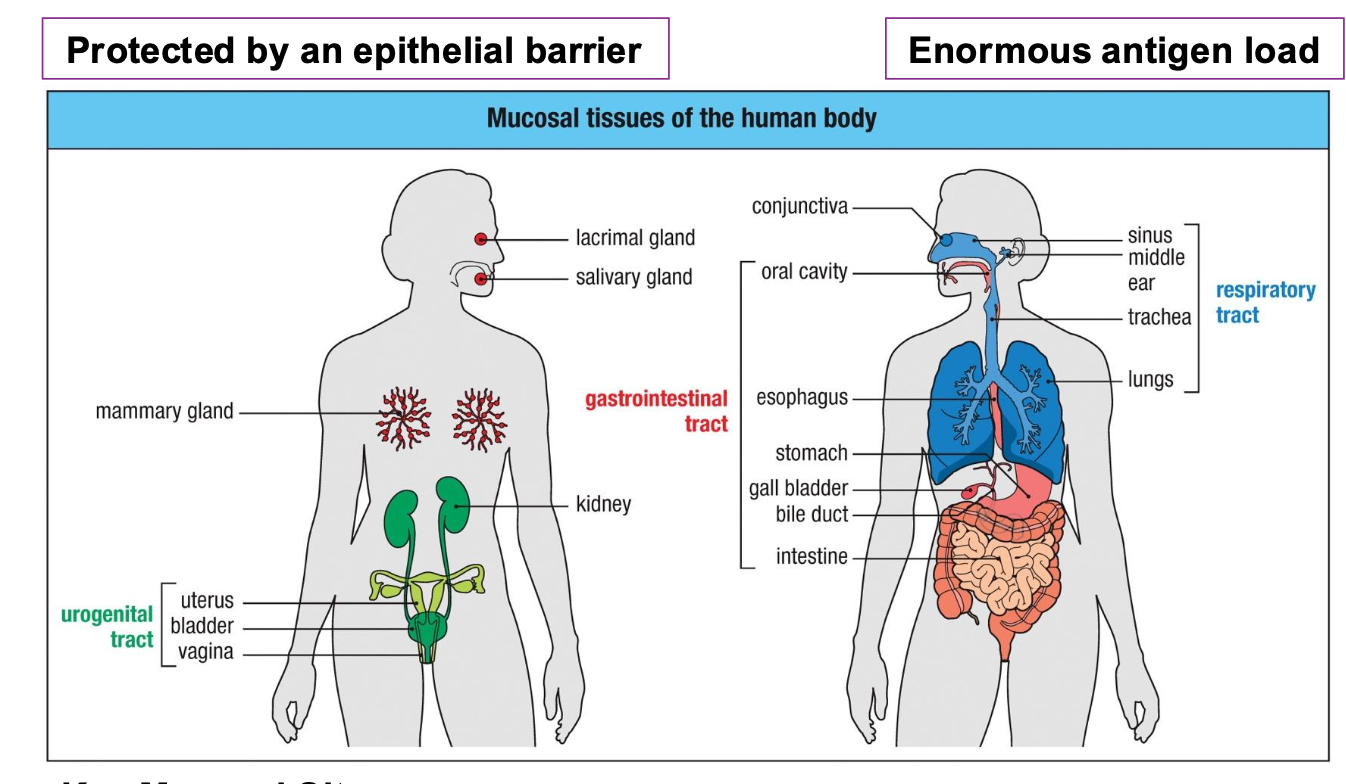
Characteristics of the mucosal immune system
Covers ~ 400 m² of surface area (vs. 2
m² of skin)
•Constantly exposed to food ags,
commensals, pathogens, and
environmental particles
•Requires tight epithelial barrier function
•Must balance tolerance (food/microbiota)
and immunity (pathogens)
Core challenges for the mucosal immune system
Enormous ag load
•Risk of inflammation → tissue damage →
loss of barrier
Key mucosal sights
•Gastrointestinal tract (the largest immune organ)
•Respiratory tract
•Urogenital tract
•Lacrimal, salivary, mammary glands
Different Types of Epithelium Line Barrier Tissue
The type of epithelium a tissue uses is the first clue to the kind of immune defenses it needs–
different types depending on the organ
All epithelia face enormous ag load – pathogens, food ags, commensal bacteria (friendly ags)
Intestine muscosae
- microvilli ↑
absorption
- Goblet cells
produce mucus
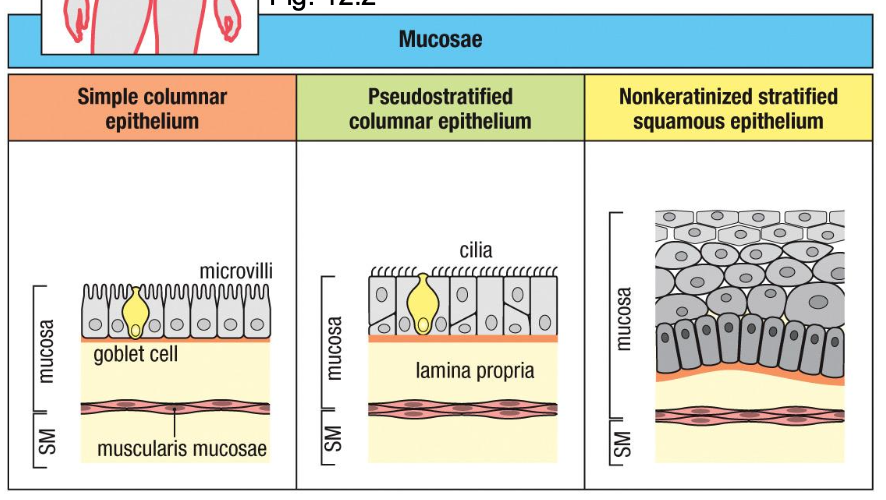
Respiratory tract mucosae
- ciliated
epithelium +
mucus clearance

Oral cavity,
esophagus mucosae
protects against
friction, non-
drying surfaces

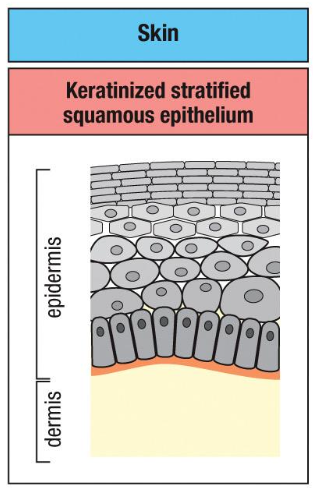
Skin
ough, water-
resistant outer layer
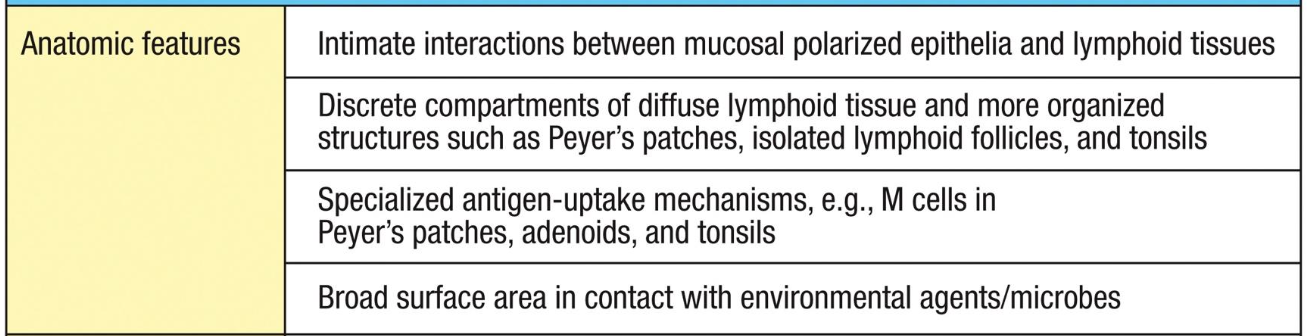
Anatomic features of the mucosal immune system
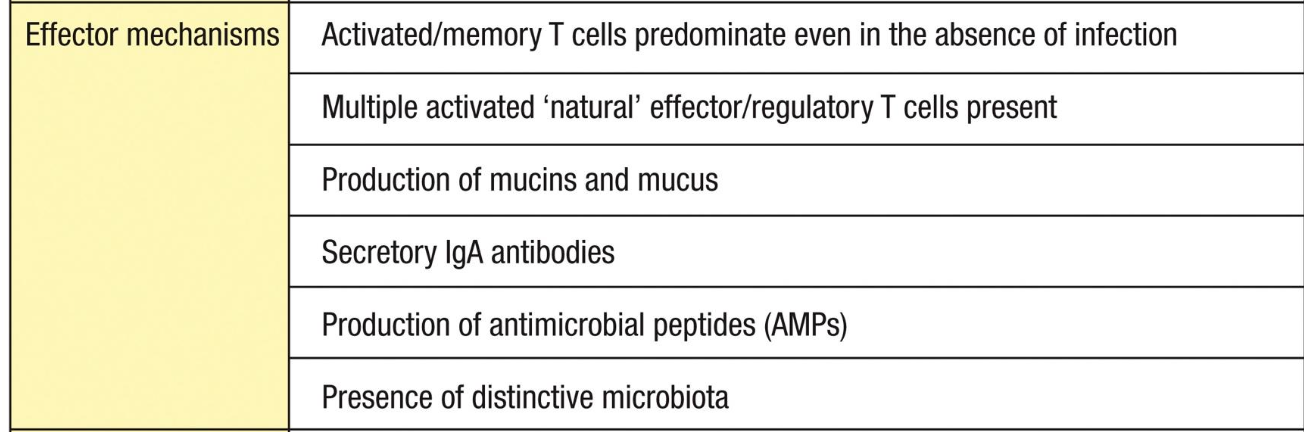
Effector mechanisms of the mucosal immune system
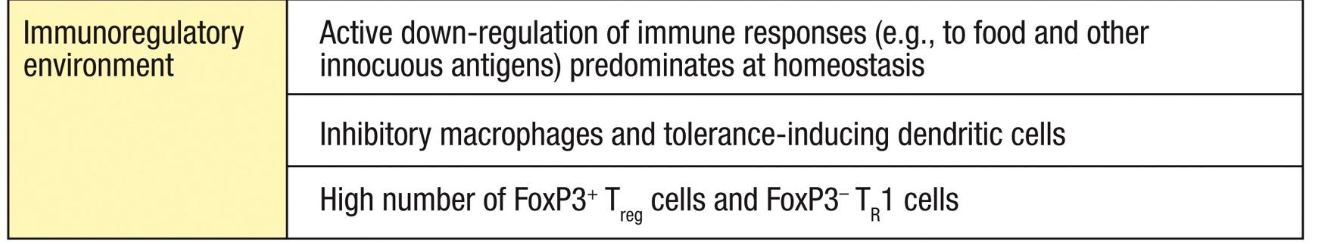
Immunoregulatory environment of the mucosal immune system
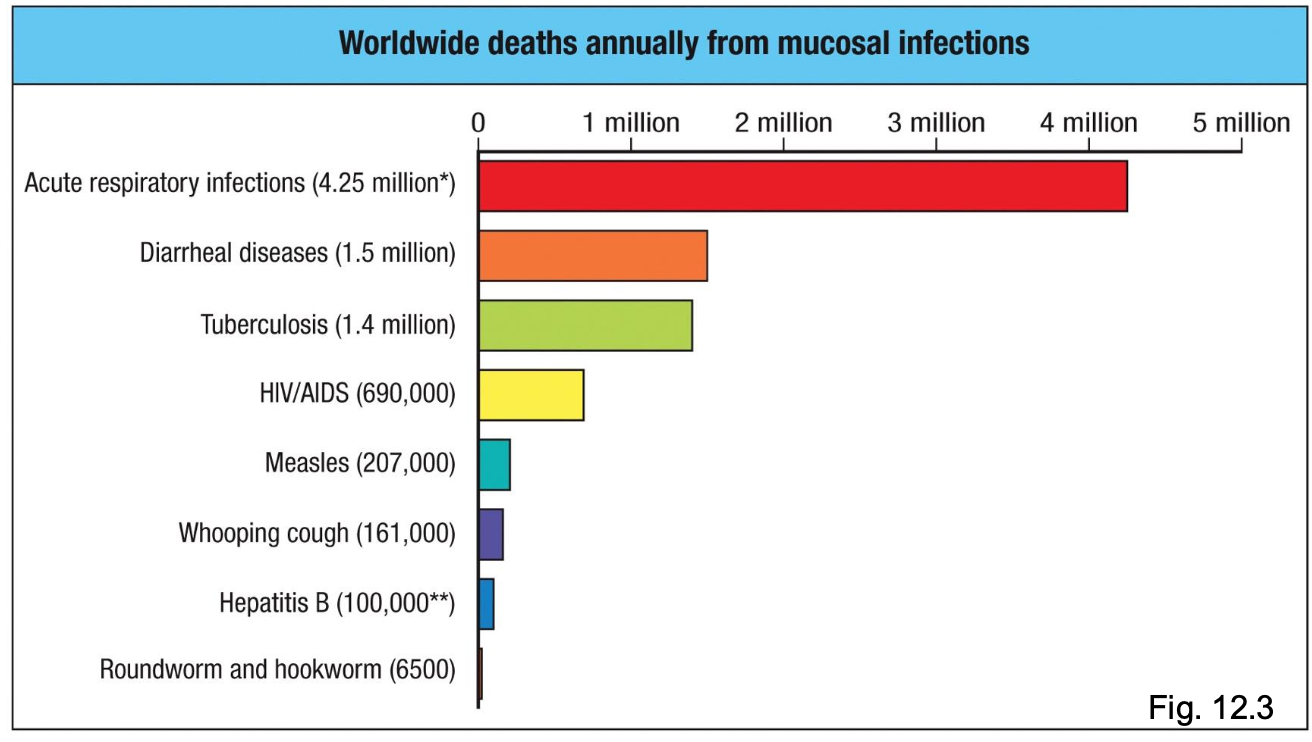
Mucosal Infections are One of the Biggest Global Health Problems
Many major pathogens enter
through mucosal surfaces:
- respiratory tract (influenza, SARS-
CoV-2, RSV, TB)
- gastrointestinal tract (cholera,
rotavirus, norovirus)
- urogenital tract (HIV, HPV,
gonorrhea, chlamydia)
Highlights why effective mucosal
immunity is critical for global health
Most infectious diseases that kill humans do so because they breach a mucosal barrier first.
What is MALT (mucosa-associated lymphoid tissue)?
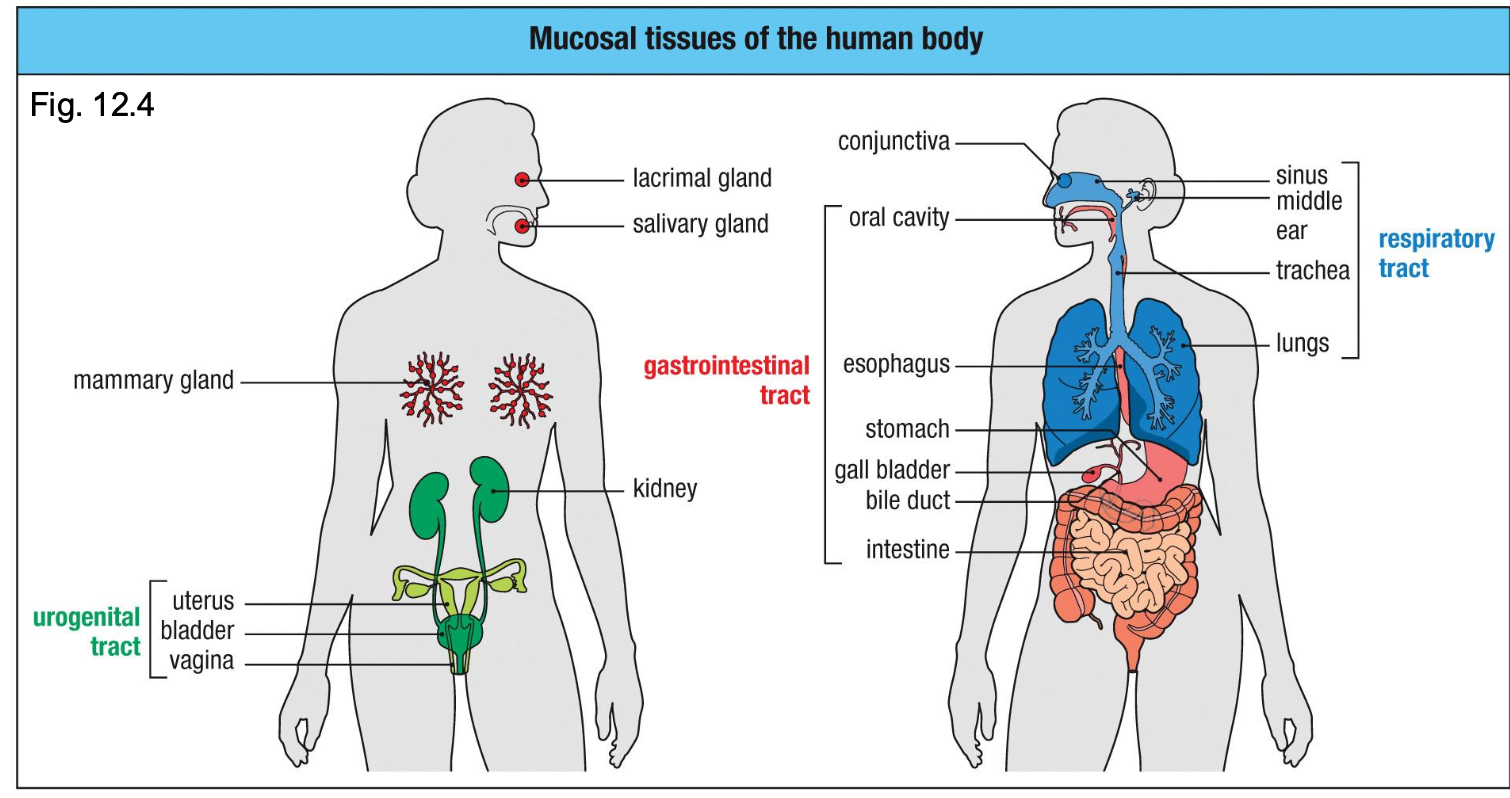
umbrella term
Also present in urogenital tract (HIV entry & STI immunity), mammary gland, conjunctiva
NALT
-tonsils, adenoids
-strong Th1/Th17
responses to
respiratory viruses
BALT
-inducible in adults
-site of TB priming in
lung
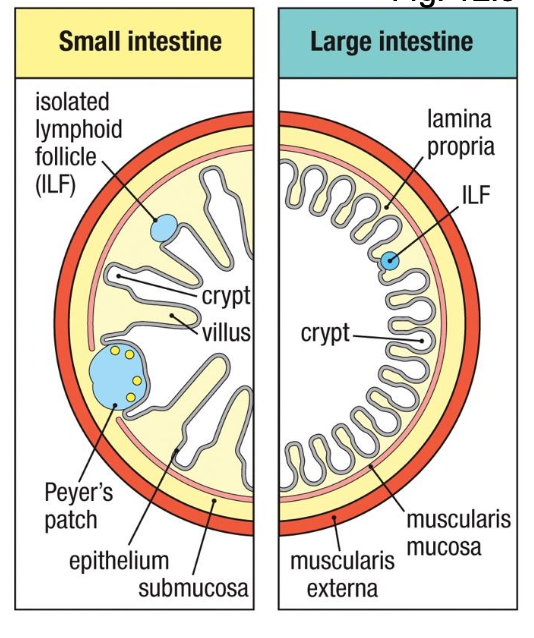
GALT
-Peyer’s patches,
isolated lymphoid
follicles, and
appendix
-oral tolerance, IgA,
defense against
enteropathogens
What is an induction site?
Where the mucosal immune response is initiated
GALT
BALT
NALT
Urogenital tract
Lacrimal glands
Salivary glands
Mammary glands
What is the effector sight?
Where the mucosal immune response is carried out
Lamina propria (large intestine) (plasma cells, T cells, ILCs)
Epithelium (intraepithelial lymphocytes, goblet cells, Paneth
cells)
IgA-producing plasma cells
Mucus secretion, AMP production, barrier fortification
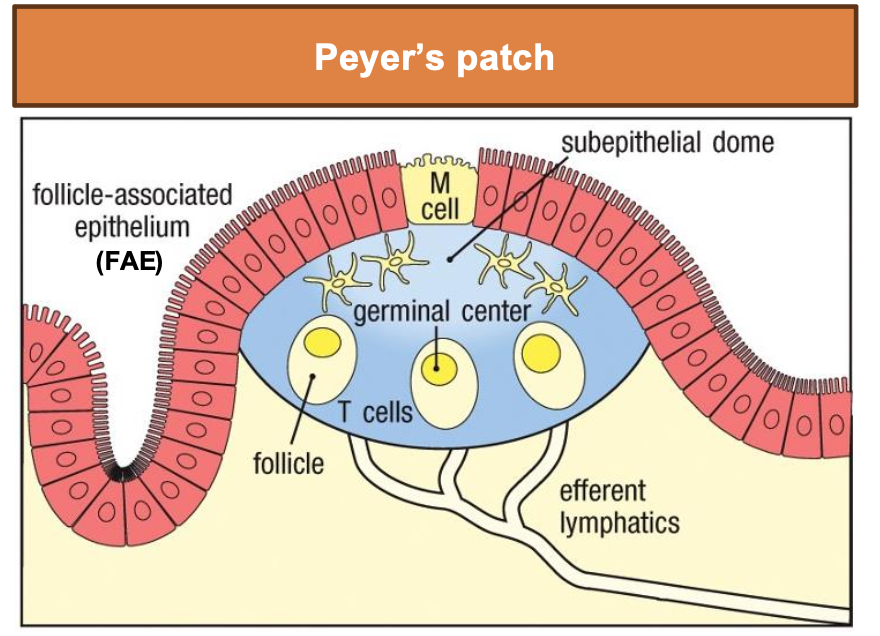
What is Peyer’s patch?
Specialized Epithelium Overlying Intestinal Lymphoid Tissues Takes Up Particulate Antigens
M cell: microfold cell – transport ag from lumen
FAE lack thick mucus → easier access for ag sampling
Subepithelial dome rich in DCs and lymphocytes
Leads to activation of B and T cells in GCs
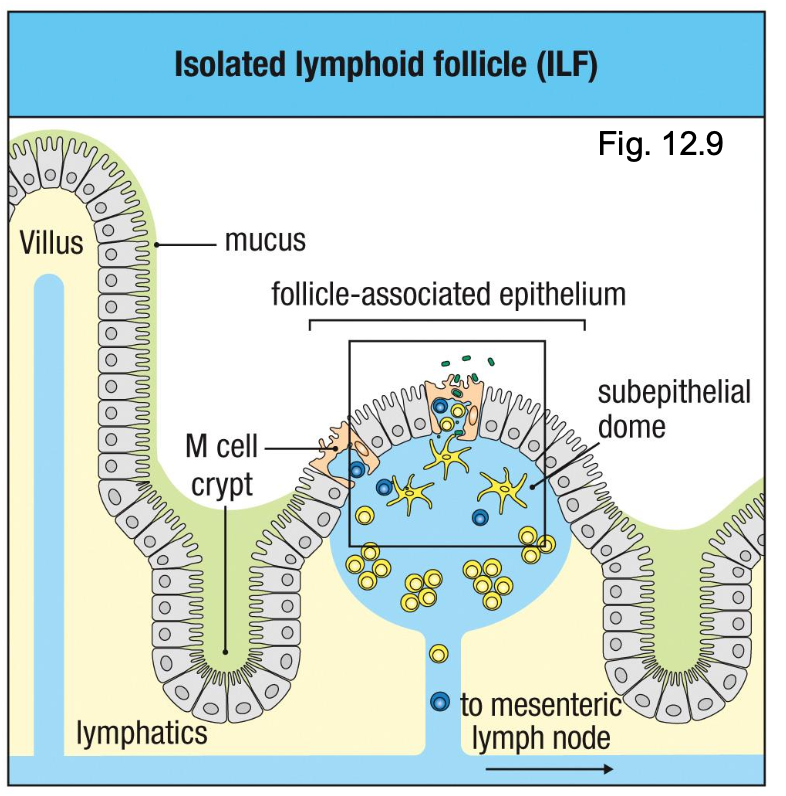
What is an isolated lymphoid follicle (ILF)?
Specialized Epithelium Overlying Intestinal Lymphoid Tissues Takes Up Particulate Antigens
Smaller inducible lymphoid structures distributed along intestine
Also contain M cells overlying lymphoid follicles
Sample ag and deliver it to underlying DCs
Connect to mesenteric lymph nodes via lymphatics
What is transcytosis?
M cells take up commensal bacteria, pathogens, and particles from the gut lumen
Transport (transcytose) them across the epithelium
Deliver ags directly to B cells, T cells, and DCs in the subepithelial dome
Enable rapid initiation of mucosal immune responses

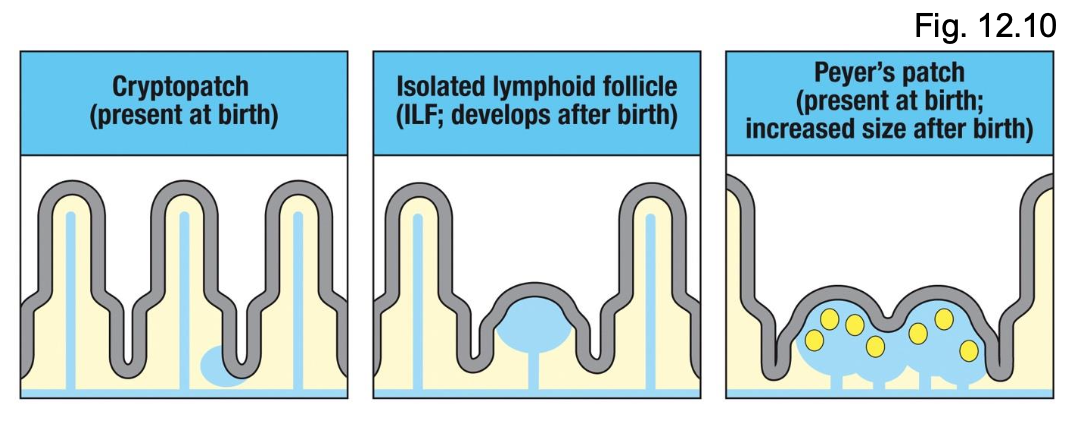
What are the developmental stages of GALT?
Cryptopatches (present at birth)
- Small clusters of DCs and lymphoid tissue-inducer (LTi) cells
ILFs
- Develop after birth; expand in response to microbial colonization
Peyer’s Patches
- Present at birth but grow and mature postnatally
- Require microbial signals for full development
What are germ free mice?
pretty much have no good or bad bacteria
Poorly developed Peyer’s patches
x ILFs
↓ IELs
↓ IgA-secreting plasma cells
↓ AMPs
↓ immune mediators (cytokines)
Maturation of GALT is Driven by
Acquisition of Commensal Microbiota
Early-life antibiotics may impair GALT development
Cesarean delivery alters initial microbial colonization
Dysbiosis can drive IBD, food allergies, asthma (systemic effects)
Small intestine effector sites
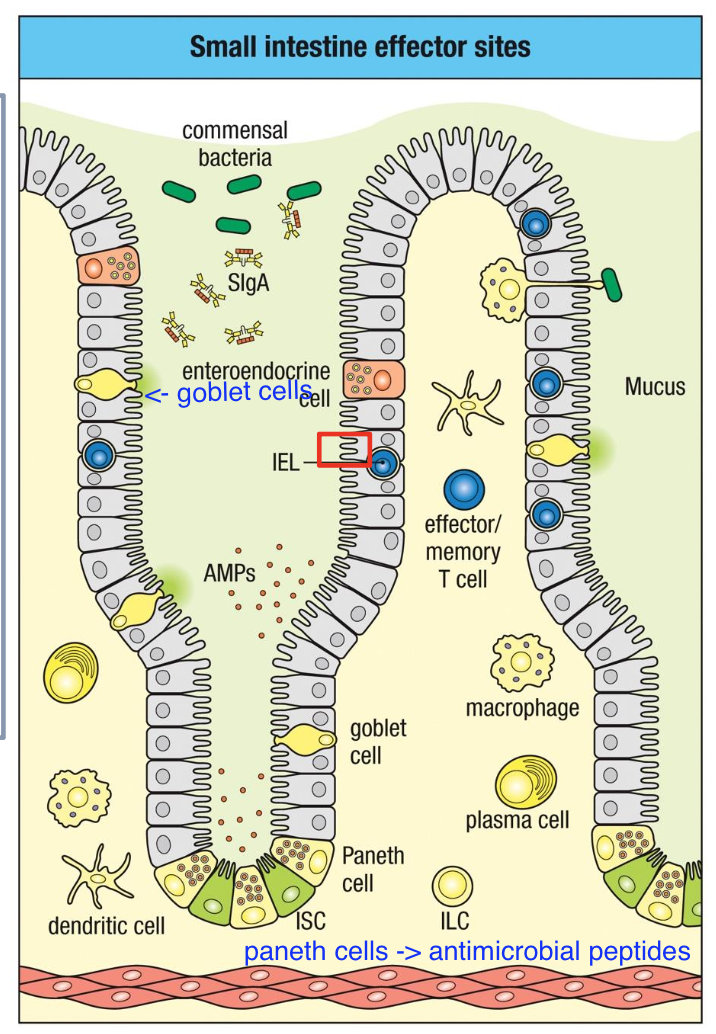
Intestinal
epithelium (single-
cell layer)
IELs (or IETs) –
mostly CD8+
No B cells
ILCs
Produce AMP,
cytokines
Interacts directly
with commensals,
sIgA, nutrients
large intestine effector site
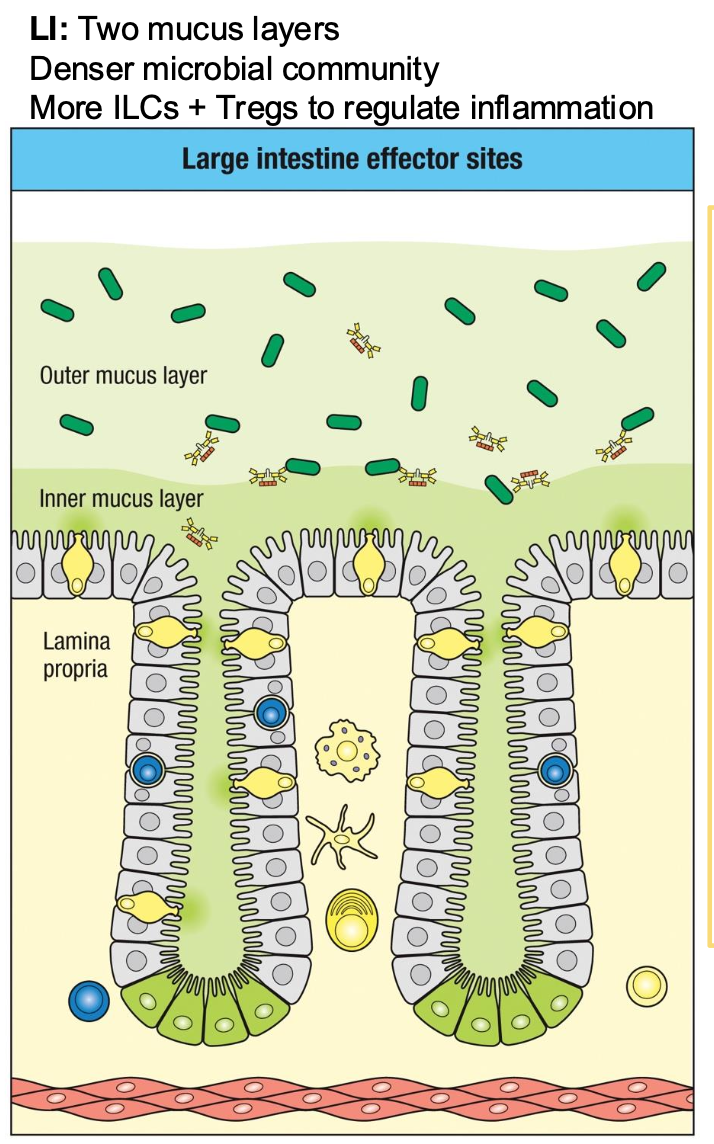
Lamina propria
Rich in Innate cells
(macrophages,
DCs, ILCs)
CD4+ and CD8+ T
cells
B cells and IgA-
secreting plasma
cells
Tregs
Site of most IgA
production and
immune effector
activity
Innate Immune Defenses of Intestinal Immune System
absorptive subsets
secretary subsets
Intestinal epithelium is not just a barrier but an active immune organ composed
of specialized innate cell types.
Absorptive subsets
Enterocytes (nutrient absorption,
sense microbes via TLRs, secrete
cytokines)
b. M cells
What makes up the Secretory subsets
Goblet cells – b. Paneth cells – AMPs
c. Enteroendocrine cells – hormones
and neuropeptides
d. Tuft cells – Cytokines and lipid
mediators – imp. in parasite
response and Type 2 immunity
Characteristics of the secretory subsets?
Viscous, slippery and sticky
• Negatively charged → retains sIgA and AMPs
• Substrate for commensals
• Major component - mucins (MUC2)
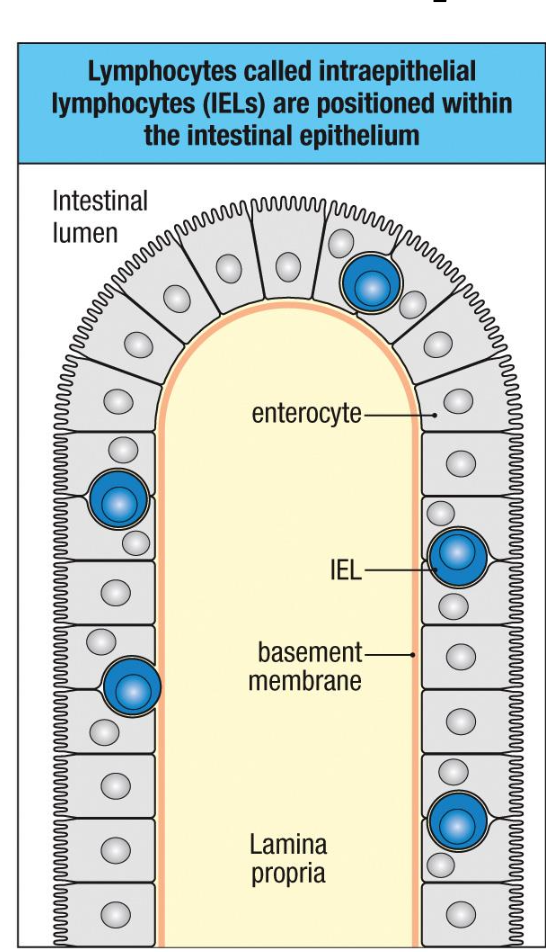
Intestinal Epithelium Contains T Cells
IELs provide rapid, front-line defense at the mucosal barrier
Include conventional CD8⁺ T cells and unconventional (innate-like) T cells
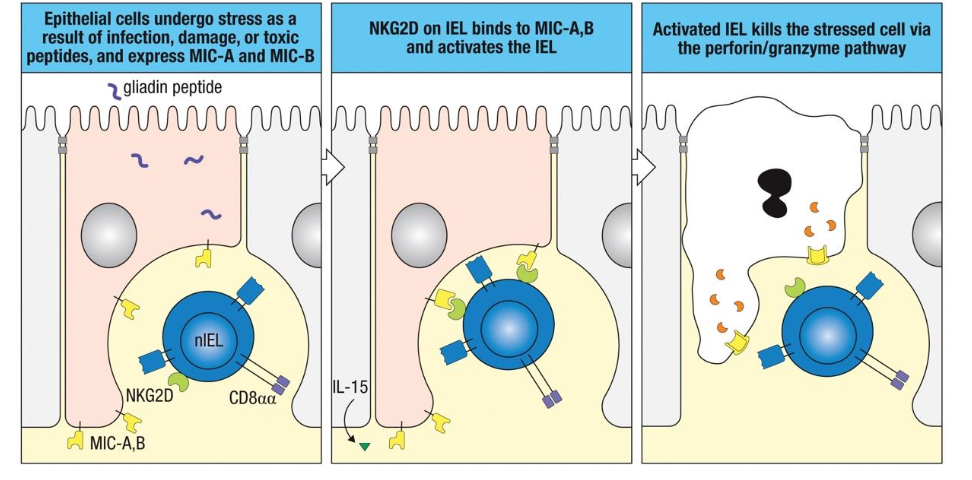
What is Type A IEL?
Function like CTL (TCR
activation → perforin,
granzyme, FasL)

Type B IEL?
Function like NK cells (act
independently of TCR
activation through NKG2D)
Loss or dysfunction of IELs
contributes to:
Celiac disease
Chronic infections
Barrier breakdown in IBD
What do ILCs do in the epithelium?
ILCs in GALT respond to Microbes that breach Epithelium
Abundant in mucosal tissues (especially intestine)
Mirror T-helper cell subsets in cytokine profiles
Activate without antigen-specific TCRs (respond
to cytokines + epithelial signals)
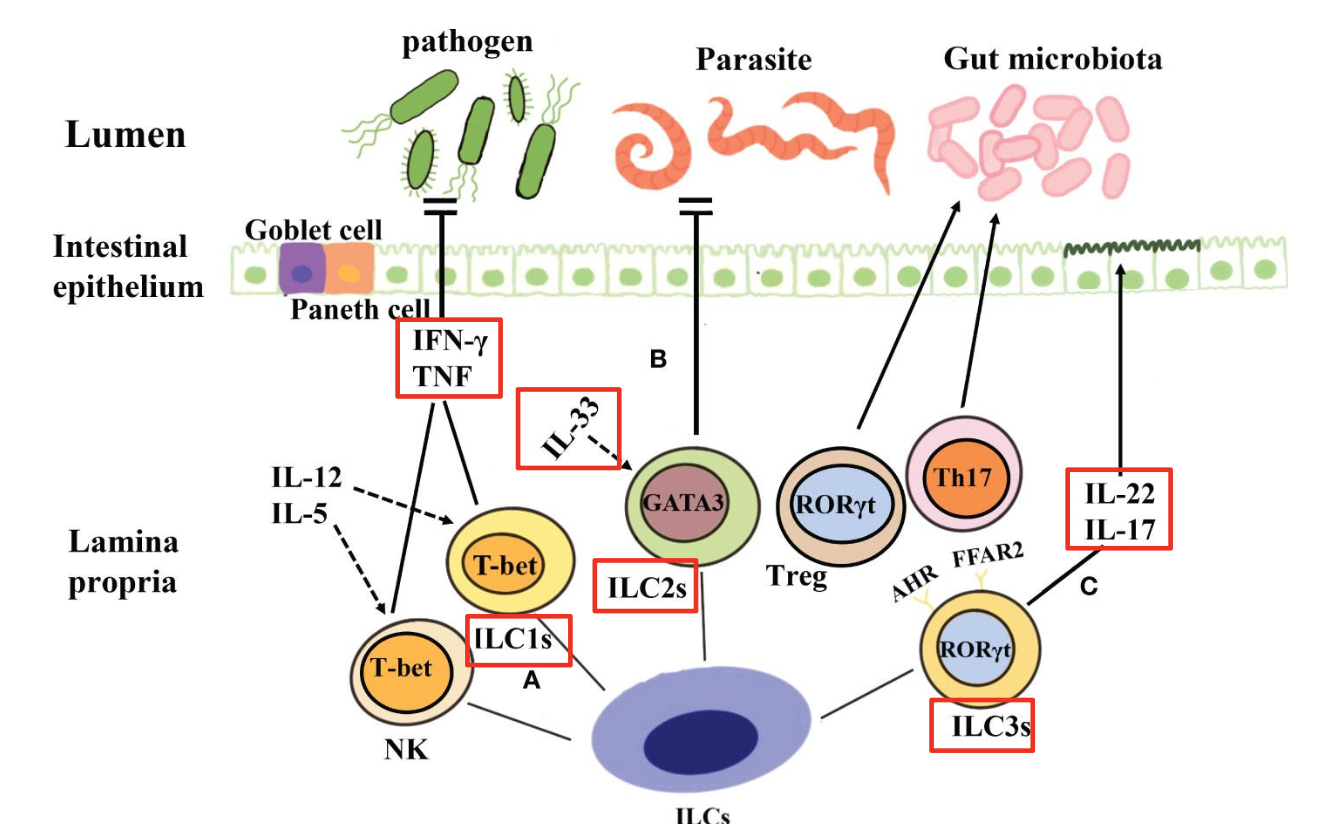
Excess ILC1
chronic inflammation, Crohn’s disease association
Excess ILC2
allergies, asthma, eosinophilic GI disease
Excess ILC3
IL-17-driven inflammation, IBD
Reduced ILC3
loss of IL-22 → weak barrier → dysbiosis
Mucosal Immune System Establishes & Maintains Tolerance to Harmless Ags
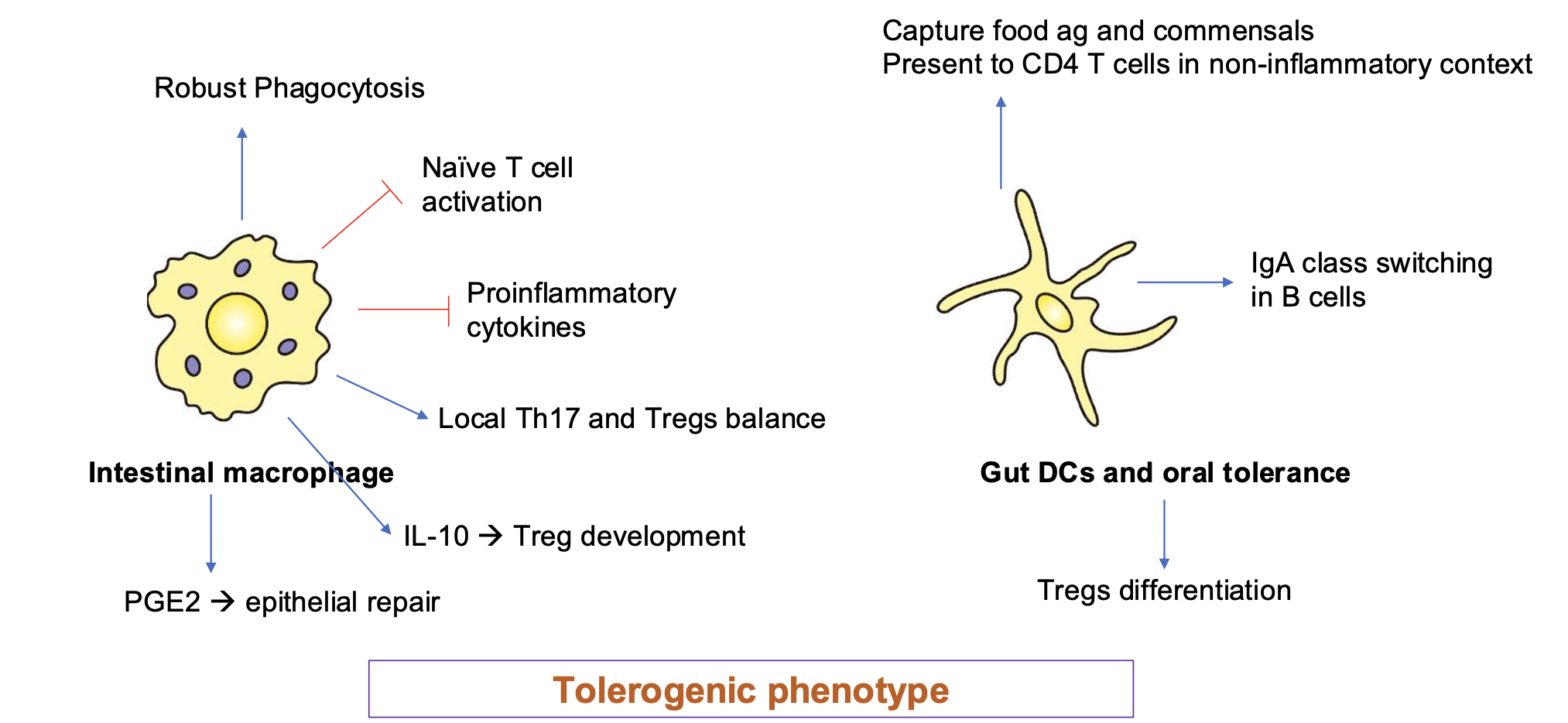
Immune Priming and Tolerance are Different Outcomes of
Intestinal Exposure to Ag
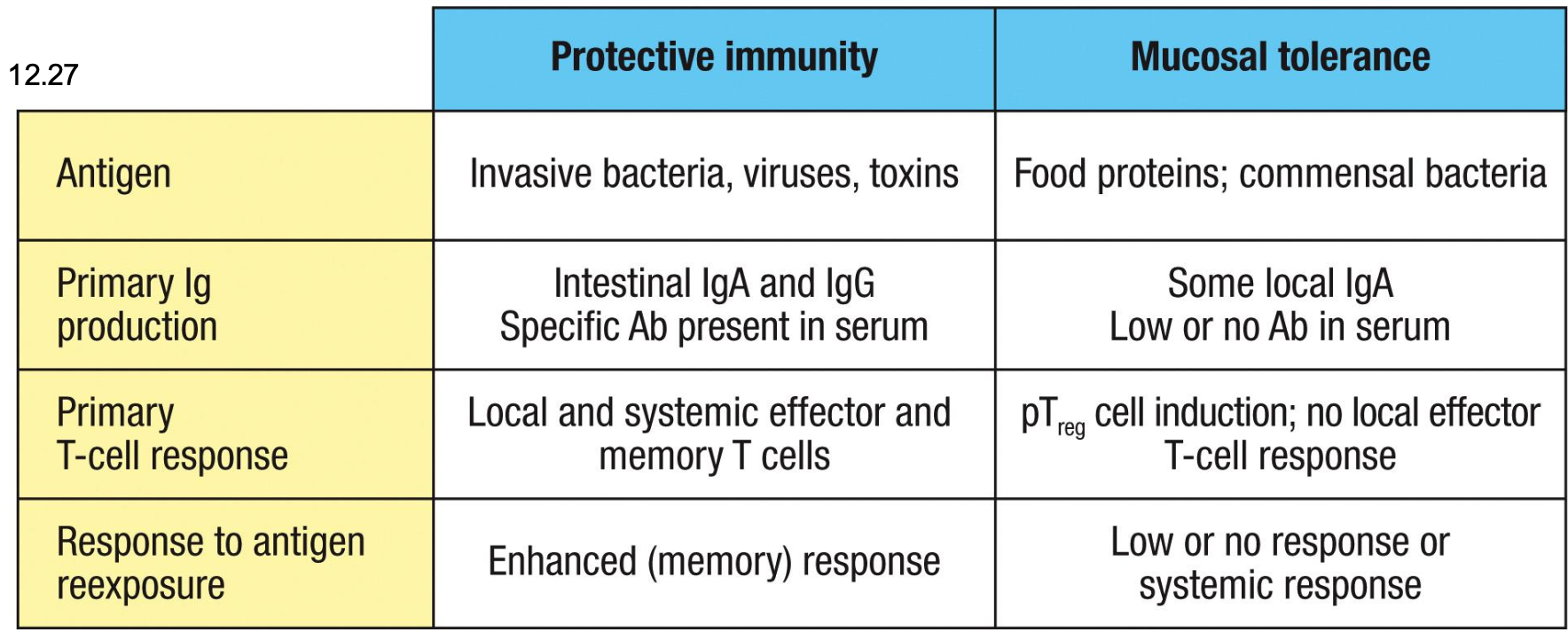
Immunity or tolerance example
The same ag delivered orally can generate immunity or tolerance depending on danger signals and
context
Pathogens → PAMPs → danger → immunity
Food + commensals → no PAMPs → steady-state DC signals → tolerance
Why commensals don’t trigger inflammation even though they
have PAMPs
1. Spatial segregation: mucus, sIgA, and AMPs keep commensals away
2. Epithelial TLRs are compartmentalized
3. Specialized tolerogenic phagocytes
4. Microbial metabolites instruct tolerance
5. Absence of contextual danger
-Danger is not just pathogen or PAMP
-True danger : PAMP/DAMP + barrier breach + inflammatory cytokines
Intestinal epithelial cells express TLRs in
strategic, non-reactive ways:
•TLR5 on basolateral surface only
•TLR9 can produce tolerogenic signals (IL-10)
when signaling from apical side
•TLR4 is often low or absent on apical
membrane
Thus, commensal PAMPs hitting apical TLRs
→ tolerance, not inflammation.
Routes of Antigen Uptake in the Intestine
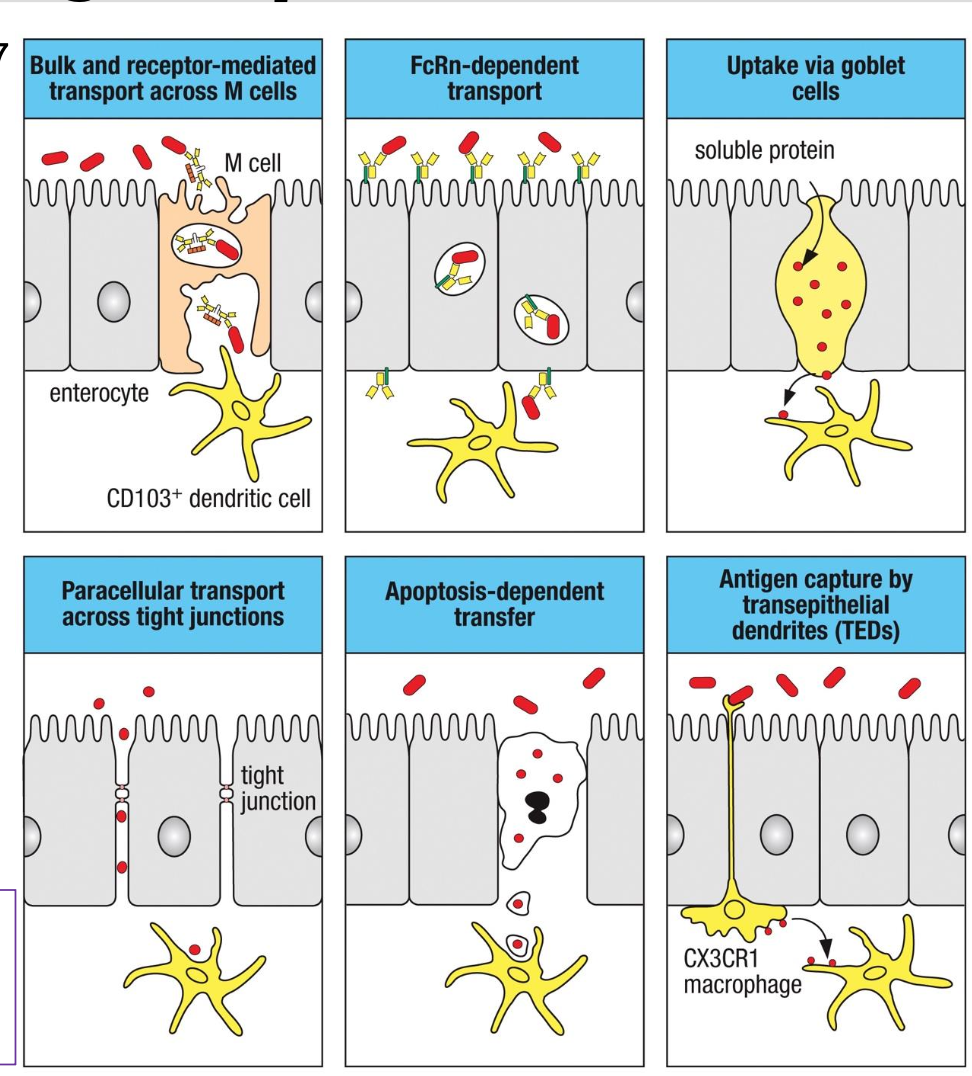
The gut does NOT rely on just one
mechanism to sample antigens; it uses
multiple, highly specialized routes
depending on ag type
What do M Cells do?
Bulk and receptor-mediated
transcytosis of microbes & particles.
Deliver ags to CD103⁺ DCs beneath
FAE
What does paracellular transport do?
Limited ag diffusion between
epithelial cells
•Occurs when tight junctions are
physiologically “open” or mildly
loosened
•Controlled, not pathological
What does FcRn: neonatal Fc receptor do?
Transports IgG-ag complexes across
enterocytes
Allows sampling of luminal antigens
bound to maternal IgG (neonates) or
endogenous IgG
What do goblet cells do?
Goblet cells can deliver soluble
proteins to underlying tolerogenic DCs.
Important for oral tolerance to food ags
What is Apoptosis-Dependent Transfer?
•IECs undergoing apoptosis release
antigenic material
•Phagocytosed by local DCs &
macrophages
What do TEDS do?
•CX3CR1⁺ macrophages extend
dendrites between epithelial cells into
the lumen
•Capture bacteria or particles directly
•Important for commensal sampling
and early pathogen sensing
Tregs During Pathogenic Exposure
Pathogens trigger innate and
adaptive immune activation
•Effector T cells, ILCs,
macrophages, and antibodies
clear infection
•Tregs prevent excessive tissue
damage during the response
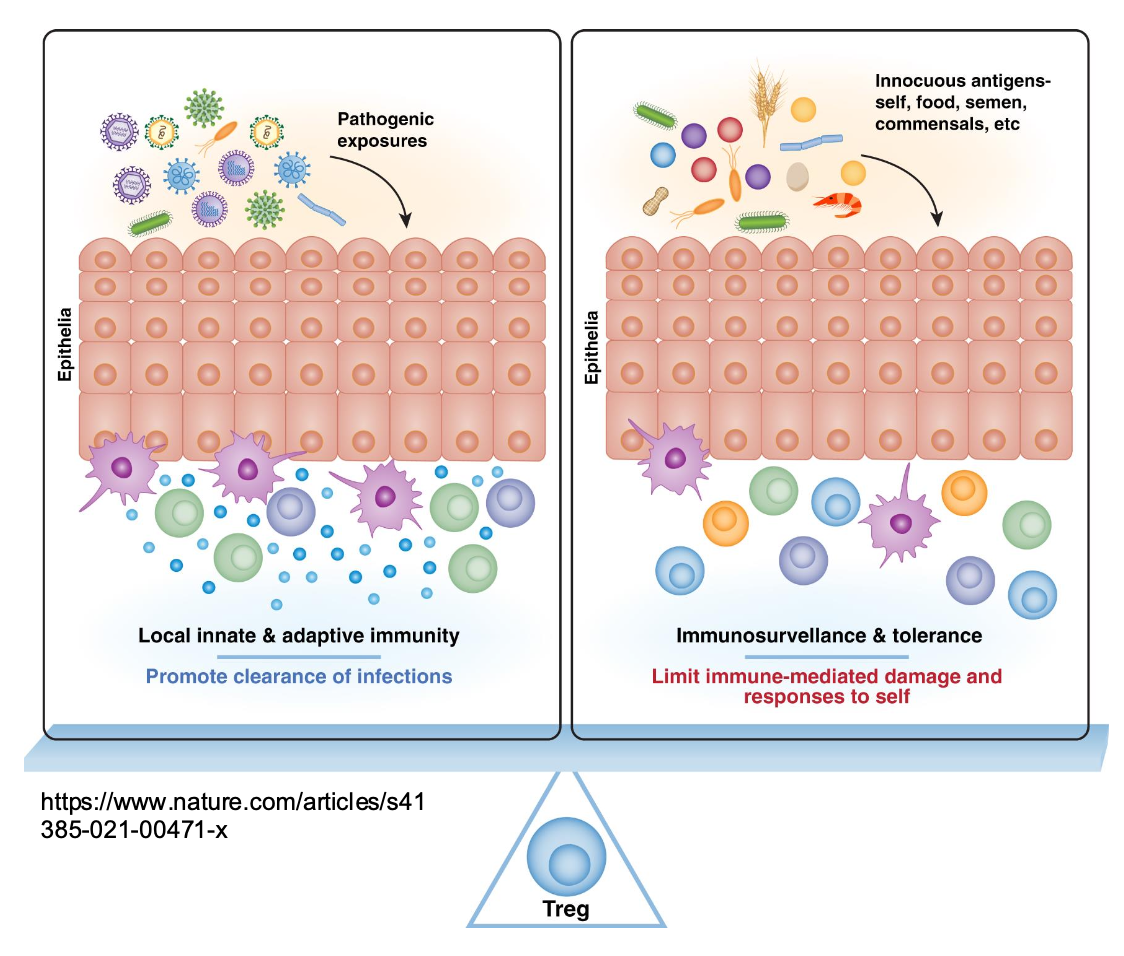
Tregs During Exposure to Innocuous Ags
•Tregs enforce tolerance and
suppress unnecessary
inflammation
•Prevent local and systemic
immune reactivity
•Protect epithelial barrier integrit

Loss of mucosal Treg function
contributes to:
•IBD
•Celiac disease
•Food allergy
•Autoimmune disorders
•Chronic infections
•HIV mucosal dysfunction
Tregs suppress effector
responses through:
•IL-10 (suppresses DCs, Th1,
Th17, macrophages)
•TGF-β (promotes IgA class
switching + epithelial repair)
•CTLA-4 (removes co-stimulatory
molecules from DCs)
•IL-2 consumption (starves
effector T cells)
•Amphiregulin (creates tissue
repair microenvironment)
Transcytosis of IgA Across Epithelia is Mediated by
Polymeric Ig Receptor (pIgR)
1. Binding (Basolateral Surface)
Dimeric IgA (with J chain) binds to pIgR
on the basolateral side of epithelial
cells.
2. Endocytosis
The pIgR–IgA complex is internalized
into the epithelial cell.
3. Transcytosis
Vesicles move the complex across the
cell toward the apical surface.
4. Release (Apical Surface)
pIgR is cleaved.
IgA is released into the lumen as
secretory IgA (sIgA).
•sIgA = IgA dimer + secretory
component (cleaved part of pIgR),
which protects IgA from proteolysis.
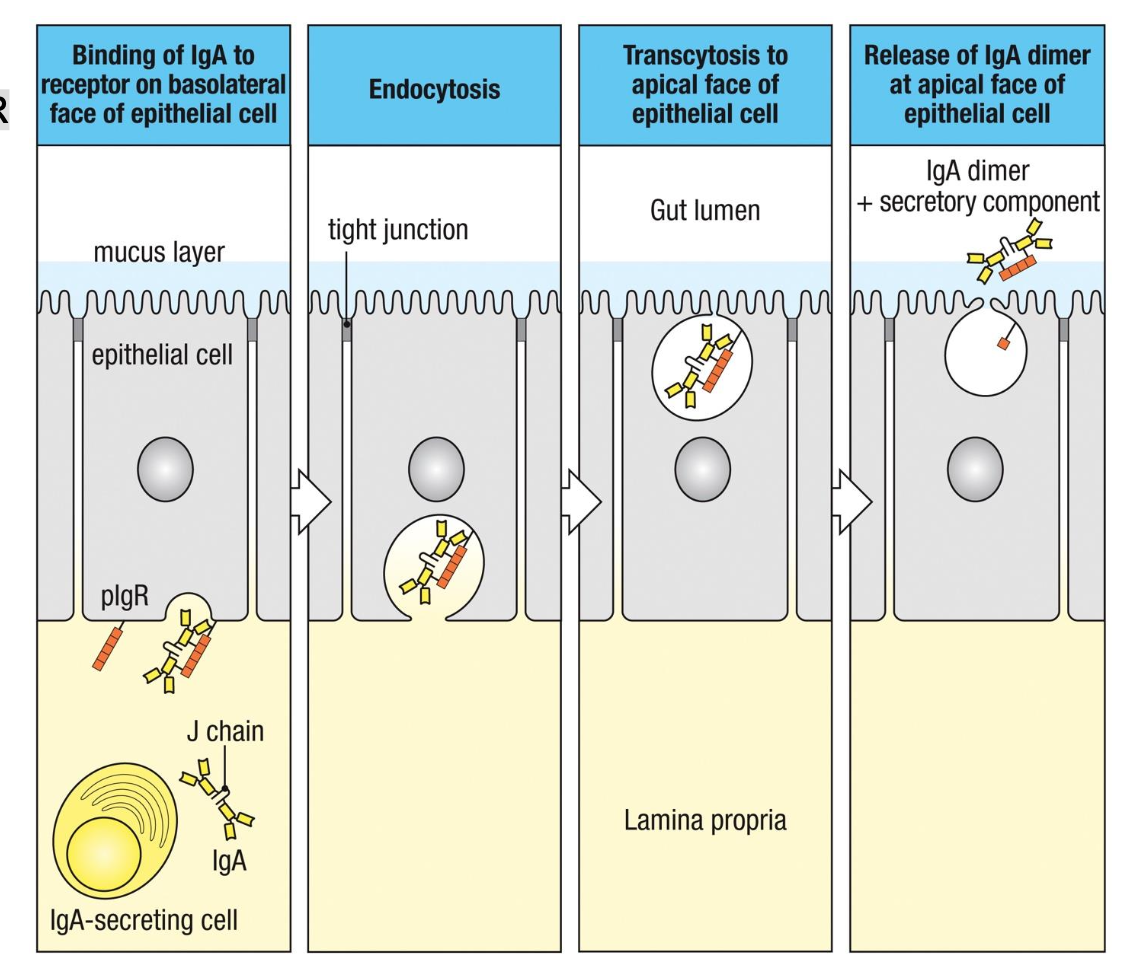
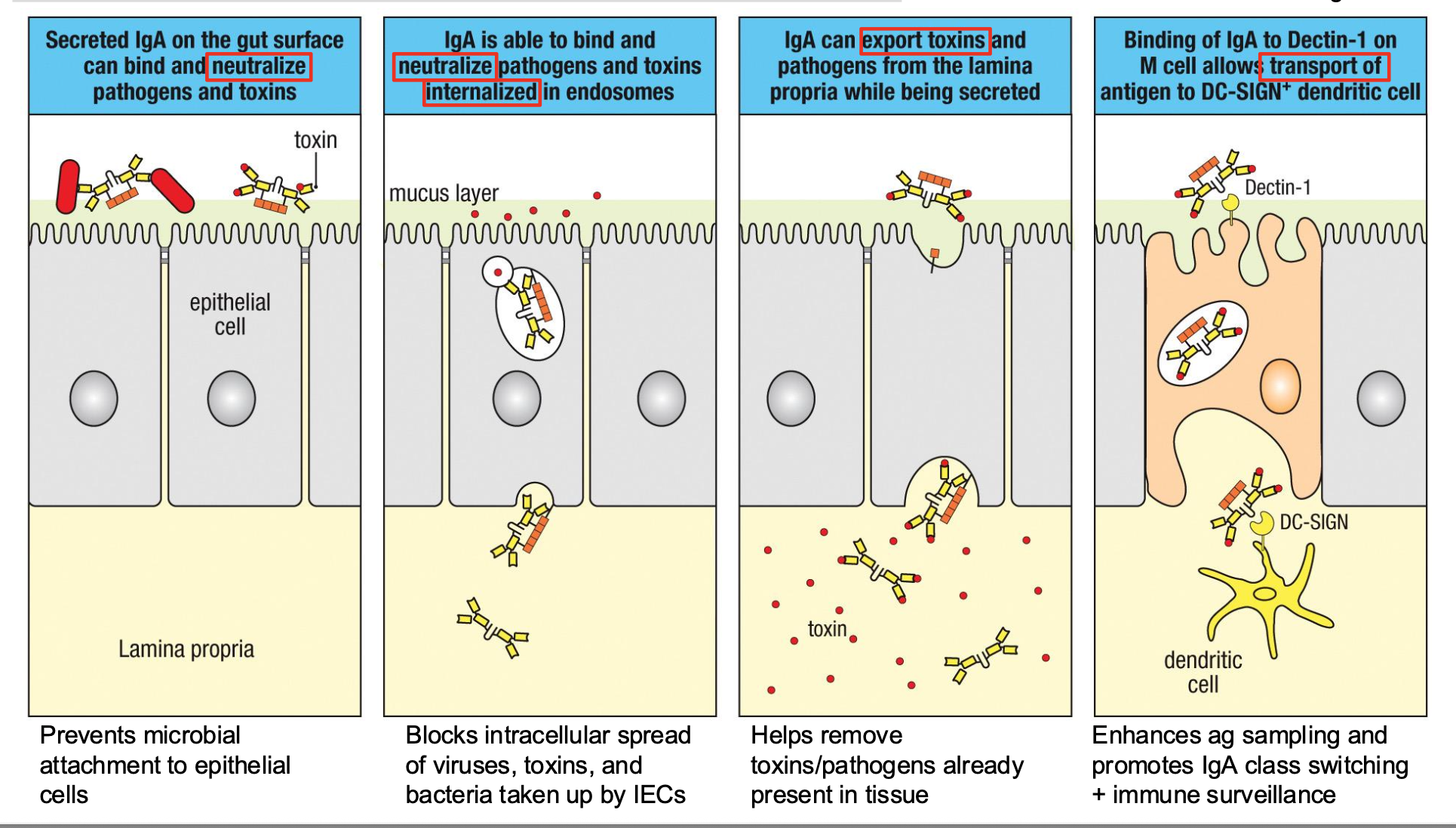
How is IgA secreted?
IgA
dimer + pIgR
secretory
component
(soluble in lumen)
Mucosal IgA has Many Functions