Electron geometry
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
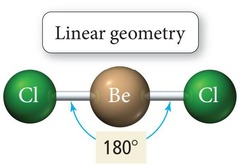
Linear Geometry
Two electron groups with 180° bond angle.
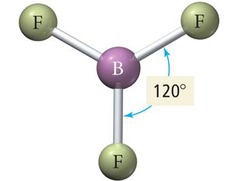
Trigonal Planar Geometry
Three electron groups at 120° angles.
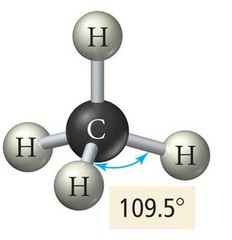
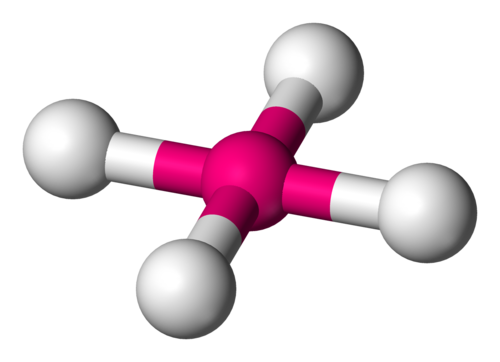
Tetrahedral Geometry
Four electron groups, bond angle 109.5°.
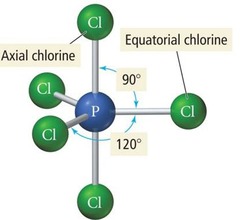
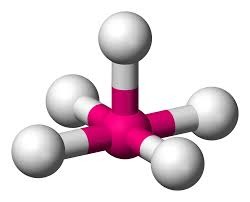
Trigonal Bipyramidal Geometry
Five electron groups; axial-equatorial bond angle is 90 degrees; eq-eq bond angle is 120 degrees
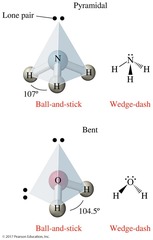
Octahedral Geometry
Six electron groups with 90° bond angles.
Pyramidal shape
four electron groups around the central atom, and one is a lone pair
effect of lone pairs - No lone pairs
109.5 degrees
effect of lone pairs - one lone pair
107 degrees
effect of lone pairs - two lone pairs
104.5 degrees
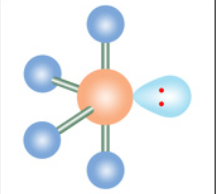
distorted tetrahedron
Five electron groups and one is a lone pairs
T-shaped geometry
Five electron groups and two are lone pairs
Linear Shape
Five electron group and three are lone pairs
Square pyramidal shape
Six electron groups and one is a lone pairs
Square planar shape
Six electron groups and two are lone pairs
Bent
Four electron groups and two are lone pairs