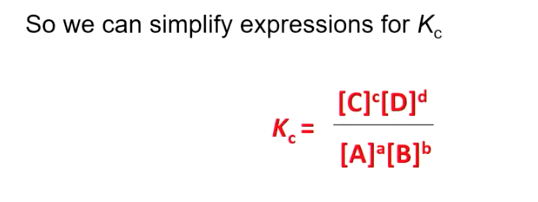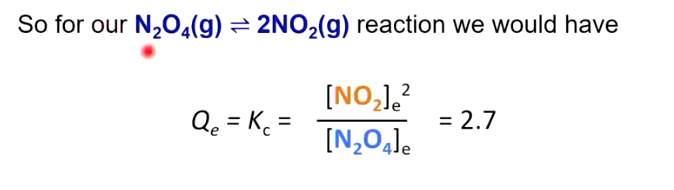CHEM191 L3 The Equilibrium Constant
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
When does Equilibrium occur
Equilibrium occurs when the rate of the forward reaction equals the rate of the reverse reaction. At this point, the concentrations of reactants and products remain constant over time.
2
New cards
Q stands for
The reaction quotient, which is a measure of the relative concentrations of reactants and products at any point in time during a reaction.
3
New cards
Kc
Kc is the equilibrium constant,
4
New cards
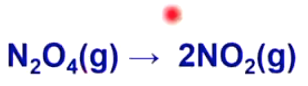
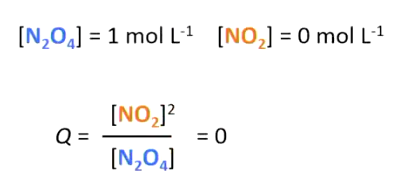
5
New cards
Unites on top mean more products made from reaction, Unit on bottom means more reactants left from reaction

6
New cards
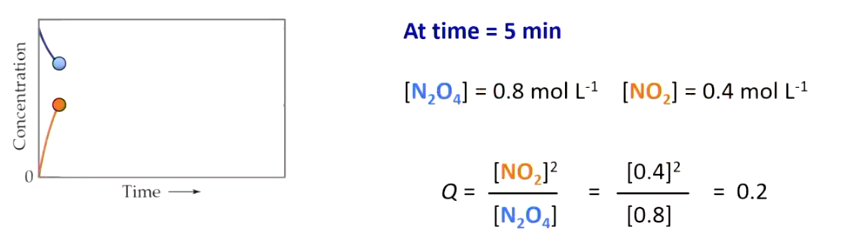
The progression

7
New cards
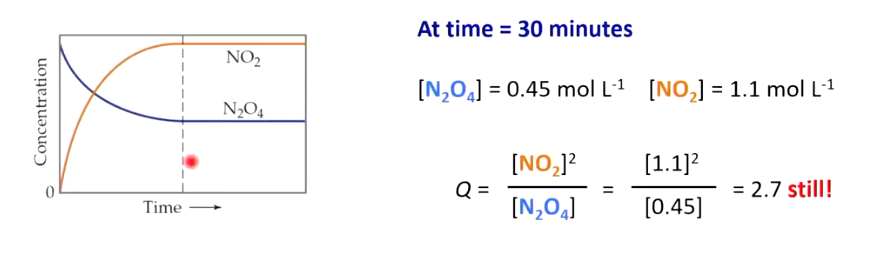
Has not gone to completion as theres still reactants in the reaction
8
New cards
The reaction going both ways at the same time, exchanging at the same rate

9
New cards