Immuno Exam 2: Immunotherapy for the Treatment of Cancer
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
3 phases of cancer development
1. elimination
2. equilibrium
3. escape
goal of immunotherapy for cancer
to educate and liberate underlying anticancer immune response in the adaptive immune system
passive immunotherapy
Antibodies or other immune system components that are made outside of the body and administered, to help them fight off cancer
active immunotherapy
Aim to stimulate effector functions of the immune system itself
goals of cancer immunotherapy
-augment the pt's own immune system to fight cancer
-overcome the mechanisms that tumors evade and suppress immune response
3 ways we can augment the pt's own immune system to fight cancer
-activate the immune system
-inhibit immunosuppression
-avoid autoimmunity
MOA of non-specific immune stimulants
rev up immune system response to all types of pathogens
examples of non-specific immune stimulants
BCG (bacillus Calmette-Guerin)
IL-2
interferon
function of naked monoclonal antibodies
bind to antigens that there are more of on the surface of cancer cells than healthy cells
MOA of monoclonal antibodies
-boost immune response against cancer cells
-block proteins that help cancer cells grow
4 ways that monoclonal antibodies boost immune response against cancer cells
-induced programmed cell death
-antibody dependent cellular toxicity (ADCC)
-complement-dependent cytotoxicity (CDC)
-antibody dependent cellular phagocytosis (ADCP)
what are conjugated monoclonal antibodies (chemotherapy)
monoclonal antibodies covalently linked to small molecular cytotoxic (chemotherapy) drugs that focus on specific cancer cell to reduce total systemic toxicity
is a mAb joined to chemotherapy
MOA of conjugated monoclonal antibodies
- mAb targets a specific cancer antigen while not harming healthy cells
◦ Potent cytotoxic small molecular agent with high systemic toxicity
◦ induces cell death after being internalized in the tumor cell and discharged from mAb
◦ Linker stable in circulation which releases the drug in neoplasms
◦ Induces higher tumor selectivity while improving the tolerability of the drug
MOA of conjugated monoclonal antibodies joined to radiotherapy
◦ Antibodyguides the radiation to the target cell
◦ Radiation also kills neighboring cancer cells that do not express the antigen, "crossfire" effect
4 limitations with monoclonal antibodies
-ADEs
-conjugated mAbs have chemo or radiation SFX
-don't work well on bulky tumors ( only access surface antigens)
-not curative- cancer develops resistance to the drug
what does BiTE stand for
bispecific T-cell engager
structure parts of BiTE antibodies and their functions
2 antibodies of interest are joined via amino acid linkage
◦ One domain interacts with T-cell
◦ One domain interacts with the desired tumor associated antigen (TAA)
MOA of BiTE antibodies
◦ T-cell perforates the tumor cell membrane
◦ Releases granzymes that induces caspase mediated apoptosis
◦ Releases cytokines
◦ Induce T-cell proliferation
major ADE of BiTE antibodies
cytokine release syndrome
4 limitations with BiTE antibodies
-cytokine release syndrome
-immune effector cell-associated neurotoxicity syndrome (ICANS)
-may need inpatient admission for observation with initial dosing to manage side effects
-many are only FDA accelerated approval
grade 1 CRS (mild)
Fever >/= 38C
grade 2 CRS (moderate)
-fever with hypotension not requiring vasopressors
or
-hypoxia requiring nasal canula
grade 3 CRS (severe)
-fever with hypotension requiring vasopressor
or
-hypoxia requiring high flow nasal canula
grade 4 (life threatening)
-fever with hypotension requiring multiple vasopressors
and/or
-hypoxia requring positive pressure ventilation
MOA of cytokine release syndrome (CRS)
-stimulus causes lysis of immune cells
-this activates macrophages and DCs
-release of pro-inflammatory cytokines
-ends in a + feedback loop
prevention of CRS
• premedications (corticosteroid, acetaminophen, diphenhydramine)
• Step up dosing (titrate up slowly)
what to do if CRS still occurs with preventative measures
Hold therapy until symptoms resolve, continue with premedication for next cycle and close monitoring inpatient
when to discontinue therapy in CRS
grade 4 or recurrent grade 3 toxicity
2 general pharmacologic treatments of CRS
• Corticosteroids
tocilizumab
MOA of immune checkpoint inhibitors (ICI) with CTLA4
blocks the binding of B7-1/B7-2 (on APC) to CTLA4 (on TC) which allows the T cells to be active and kill tumor cells
functions of checkpoint proteins
help keep the body's immune responses in check
T cells can be activated when...
• T-cell receptor (TCR) binds to antigen and MHC on the APC
AND
• CD28 binds to B7-1/B7-2 on the APC
when are T cells inactive and not able to kill tumor cells
with the binding of B7-1/B7-2 to CTLA4
MOA of immune checkpoint inhibitors (ICI) with PDL1/PD1
blocks the binding of PD-L1 to PD-1 which allows the T cells to stay active and kill tumor cells
PDL-1 vs PD-1 and function
PDL-1 is on tumor cells and PD-1 is on T cells
-the binding of PD-L1 to PD-1 keeps T cells from killing tumor cells in the body (regulation of immune system)
how do you explain immune checkpoint inhibitors to patients
Gas and brake pedal analogy
◦ Pressing the gas pedal = restoring T-cell activity and starting immune response against tumor
◦ Brake pedal = immune checkpoints will inhibit T-cell activity so we want immune checkpoint inhibitors (cutting breaks)
high PD-L1 expression
-PD-L1 is a biomarker
-high amount --> improved response rates, progression free survival, overall survival
MSI-H (microsatellite instability-high) as biomarker for ICIs
high MSI-H --> higher mutational burden--> upregulated expression of programmed cell death -1 (PD-1) and its ligand (PD-L1)
-MSI-H/dMMR predictive biomarker for immune checkpoint blockade in different cancer types
what is irAE
immune related adverse effects
side effect of immune checkpoint inhibitors
irAEs of CTLA-4
colitis and thyroiditis
irAEs of PD-1
pneumonitis and thyroiditis
timing and speed of treatment of irAEs
early recognition and prompt intervention critical for effective management and optimal clinical outcomes
why do ICI's cause irAEs? (2 functions)
-ICIs promote activation and expansion of T cells
-ICIs can affect any organ due to ability of T cells infiltrating most organs
monitoring parameters for immune related AEs
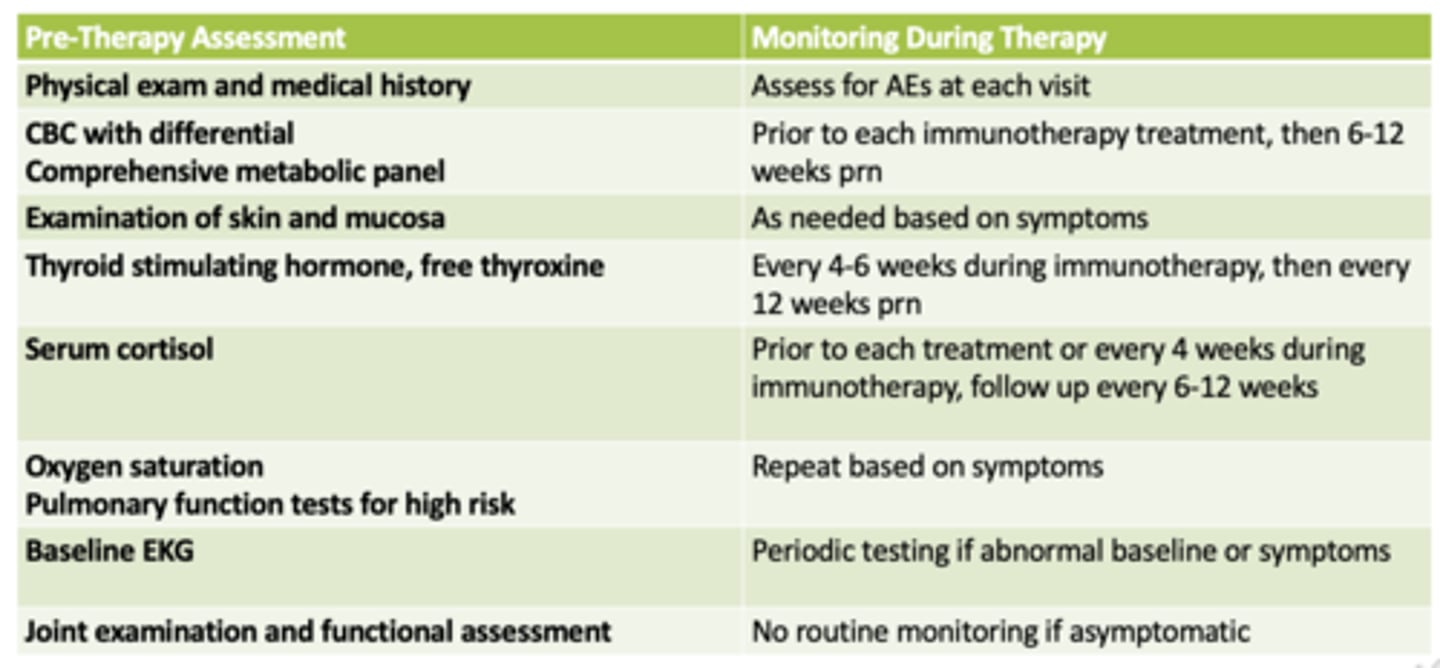
how are hypothyroid and endocrine irAEs managed
with hormonal supplementation without steroids
what's the main treatment of most irAEs
corticosteroids
tapering of cortidosteroids for treatment of irAEs
taper off of them for >4 weeks once symptoms resolve
patients with a pre-existing autoimmune condition and ICIs/irAEs
◦ higher risk of exacerbating underlying condition
◦ Allows for dose of prednisone < 10 mg daily (more could interfere with ICI efficacy)
management and ICI therapy of grade 1 irAEs (asymptomatic or mild)
observation
continue ICI with close monitoring
management and ICI therapy of grade 2 irAEs
-0.5-1mg/kg/day of prednisone or equivalent
-temporary hold on ICI; resume when grade
management and ICI therapy of grade 3 irAEs
management:
-treat inpatient
-1-2mg/kg/day of prednisone or methylprednisone
-if no response in 48-72 hours consider additional therapy
ICI: temporary hold until grade 1 or less of reaction
management and ICI therapy of grade 4 irAEs
management;
-treat inpatient +/- ICU
-1-2mg/kg/day of prednisone or methylprednisone
-if no response in 48-72 hours consider additional therapy
ICI: permanent discontinuation
exception: endocrinopathies managed by hormone replacement
pseudoprogression with ICIs
tell the patient this is normal
disease can get worse (due to inflammation and TC infiltration of tumors) before getting better
what type of therapy are chimeric antigen receptors (CAR)
adoptive T cell therapy
6 steps for CAR T-cell therapy
1. leukapheresis: blood drawn and T cells and removed, rest of blood is returned
2. collected TCs are sent to lab and receptors are added to create patient-specific CAR T-cell therapies
3. pt prepares for CAR TC therapy by getting low dose chemotherapy
4. infusion of pt's programmed T cells
5. monitor for side effects
6. continues monitoring for side effects and response to therapy
4 limitations of CAR-T cell therapy
1. antigen loss- cancer cells decrease antigen expression
2. T-cell exhaustion- fail to provide long-term protection
3. on-target/off-target tumor effect- B cell depletion
4. toxicities (acute or delayed)
what can CAR TC therapy cause
it can cause CRS (cytokine release syndrome) and ICANS (immune effector cell-associated neurotoxicity syndrome)
types of acute CAR TC toxicity
1. CRS
2. neurotroxicity
-immune effector cell-associated encephalopathy (ICE)
- immune effector cell-associated neurotoxicity syndrome (ICANS)
delayed CAR TC toxicities
-prolonged cytopenias
-opportunitistic infections
-on-target but off-target tumor effect
-B cell aplasia
-hypogammaglobulinemia
management of grade 1 CRS with CAR TC therapy
Tocilizumab: consider if CRS > 3 days with significant symptoms &/or morbidities
Supportive Care: antibiotics, G-CSF if neutropenic, symptomatic management
management of grade 2 CRS with CAR TC therapy
Tocilizumab: 8 mg/kg IV over 1 hour, may repeat in 8 hrs, no more than 3 doses/24 hrs
Corticosteroids: for persistent refractory hypotension after 1-2 doses of tocilizumab
Supportive Care: add vasopressors for refractory hypotension, transfer to ICU, hemodynamic monitoring
management of grade 3 CRS with CAR TC therapy
Tocilizumab: same as grade 2
Corticosteroids: dexamethasone 10 mg IV every 6 hours
Supportive Care: transfer to ICU
management of grade 4 CRS with CAR TC therapy
Tocilizumab: Yes as with grade 2
Corticosteroids: dexamethasone 10 mg IV every 6 hours. If refractory, consider methylprednisolone 1000 mg/day IV
Supportive Care: ICU care
management of neurotoxicty with CAR TC therapy grade 1 (with and without CRS)
no CRS: supprtive care
w/ CRS: tocilizumab
management of neurotixicty with CAR TC therapy grade 2 (with and without CRS)
no CRS
-supportive care
-dexmethasone 10mg IV q6 hours or methylprednisone 1mg/kg q 12 hours
w/ CRS
-tocilizumab
-consider ICU
management of neurotoxicty with CAR TC therapy grade 3 (with and without CRS)
no CRS:
-ICU
-dexamethasone 10mg Iv q 6h or methylprednisone 1mg/kg q 12 hours
-CT or MRI q 2-3 days if persistent
w/ CRS
-tocilizumab
-consider ICU
management of neurotoxicty with CAR TC therapy grade 4 (with and without CRS)
no CRS:
-ICU, consider ventilation
-high dose steroids
-CT or MRI q 2-3 days
-treat seizures
w/ CRS:
-tocilizumab
-consider ICU
pros and cons of tocilizumab
pro: Early administration may prevent high-grade CRS while maintaining efficacy of CAR T-cell therapy
con: early administration may predispose to neurologic toxicity
-Does not cross blood brain barrier and may increase IL-6 concentration in the
CNS
pros and cons of corticosteroids in CAR TC toxicities
con: high dose can decrease efficacy of CAR TC therapy
pro: reduced neurotoxicity if recieved at grade 1 vs more severe grades
function of therapeutic cancer vaccines
§ Increase presentation of tumor-associated antigens (TAAs) to the immune system
§ Increase activation of tumor-specific T cells and B cells
MOA of therapeutic cancer vaccines (example of prostate cancer vaccine)
1. pt's peripheral blood cells + APCs + TCs are harvested
2. harvested cells cultured ex vivo with recominant fusion protein composed of prostatic acid phosphatase and GM-CSF)
3. mature APCs reinfused into the patient to stimulate CD4+ and CD8+ cells
4. triggers immune response against cancer cells
treatment course of cancer vaccine
• 3 doses administered at 2 weeks interval
• Each dose is manufactured 3 days prior to infusion
• 1st infusion primes the anti-tumor immune response
• Subsequent 2 infusions boost the response
T-VEC (talimogene laherparepvec, Imlygic)
-first in class oncolytic immunotherapy
-contains a genetically modified version of herpes simplex virus that can selectively replicate in the tumor and cause cell destruction
-contains a gene for granulocyte-macrophage colony stimulating factor (GM-CSF) to attract dendritic cells once the rupture of cancerl cells releases tumor-derivced antigens
all together stimulates a system-wide immune response