Intermolecular forces
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
Intermolecular forces
Forces between molecules or ions.
Ionic compounds
- Forces between the positive and negative
- Ionic forces are present in ionic compounds
Covalent compounds
Have no charges but can have what type of forces (2) and bonds (1)?
1. Dispersion forces
2. Dipole-dipole forces
3. H-Bonds (hydrogen bonds)
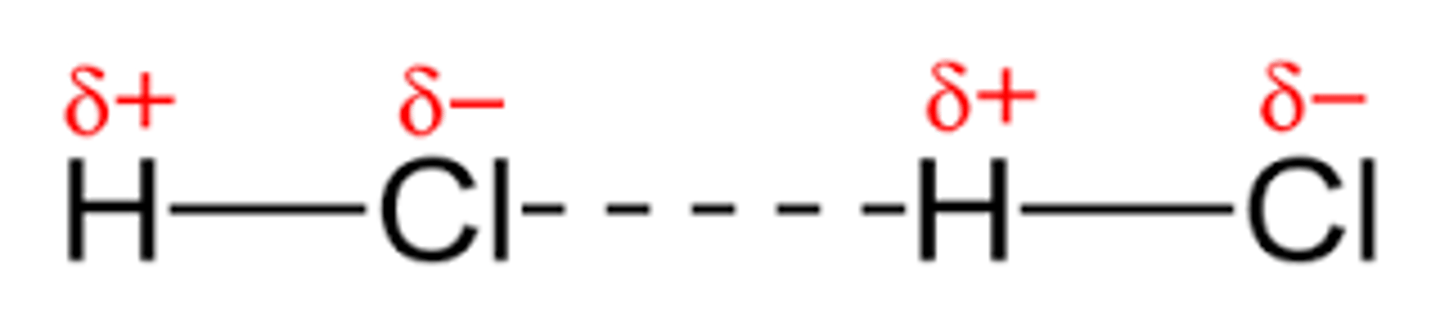
Polar molecules have what type of intermolecular forces?
dipole-dipole
Non-polar molecules have what type of intermolecular forces?
Dispersion forces
Polar ? are stronger than ?
Polar molecules are stronger than dipole dipole intermolecular forces
Types of intermolecular forces
1. Dispersion
2. Dipole-dipole
3. H- Bonds (hydrogen)
Dipole-dipole attraction
Forces of attraction between polar molecules as a result of the dipole moment within each molecule
Hydrogen bond
1. the dipole-dipole attraction between polar molecules containing these three types of polar bonds (fluorine, oxygen or nitrogen)
2. Stronger than dipole-dipole
Hydrogen bonds are stronger than
dipole-dipole
H20 Bond forces
1. dipole- dipole (the dipole-dipole attractions between polar molecules containing hydrogen and (N, O or F)
2. Hydrogen bond - a hydrogen bond is a dipole dipole attraction
3. Dispersion
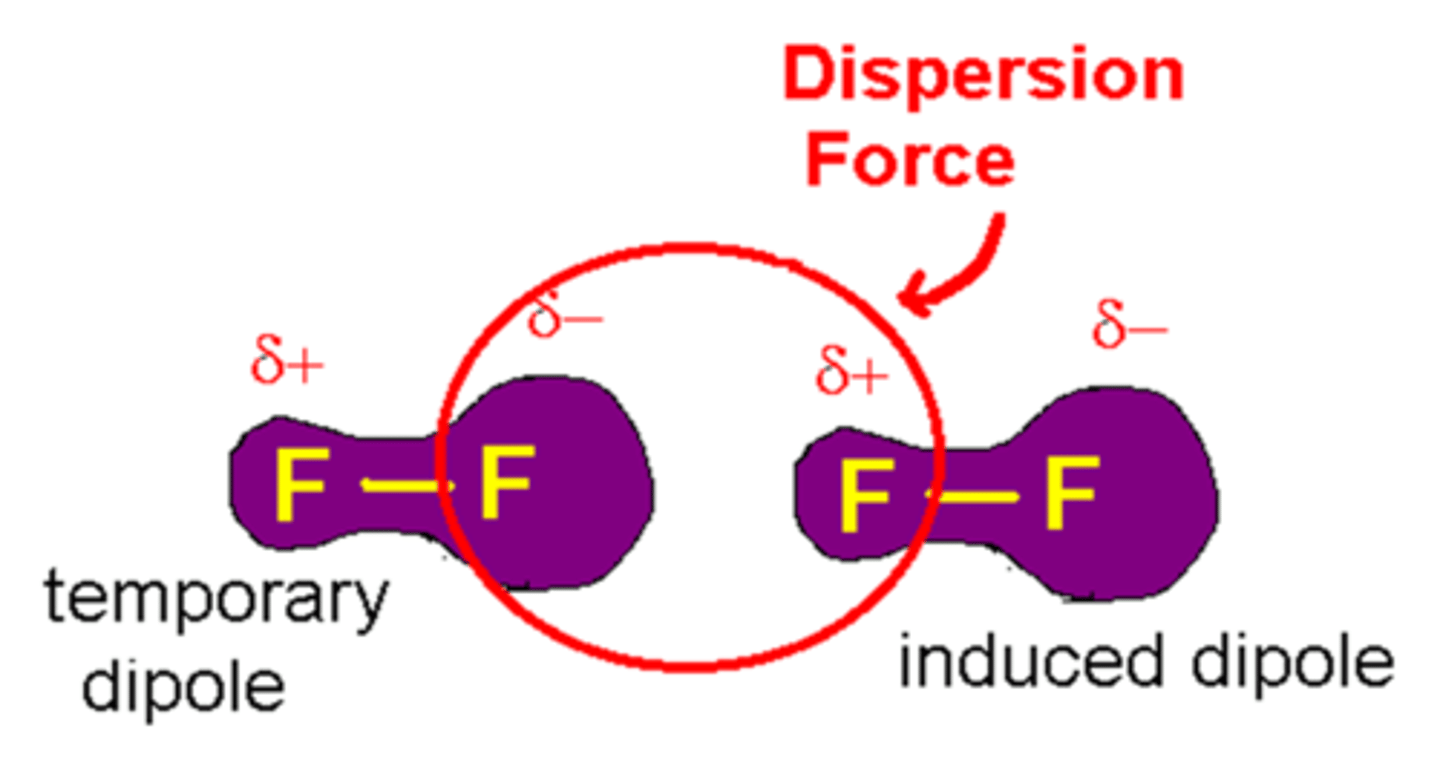
London dispersion factors
- Forces that exist between nonpolar molecules and also between noble gas molecules
- Electrons are in motion around the nucleus so an even distribution is not true all the time.
- Atoms can develop an instantaneous dipolar arrangement of charge. This instantaneous dipole can induce a similar dipole in a nearby atom
- Interaction is weak and short-lived
The strength of London dispersion depends on
- Strength of attractions depend on the molar mass of the substance.
- Larger size means more electrons are available to form dipoles
Dispersion forces are weaker than
dipole dipole
List in order of least strongest to stongest...
Dispersion
Dipole Dipole
H-bonds
Dispersion < Dipole-Dipole < H-bond
Dispersion forces are present in
Non polar molecules
ex. CO2, CH4, Noble gases (have dispersion forces between atoms when come together, don't make compounds)
Hydrogen bonds are between molecules of H and
Between H and N,O, or F
ex. H20, NH3, HF
start to share electrons
Dipole-Dipole are formed between
Polar molecules
ex. H20, HCL
Ionic compounds have what type of forces?
Ionic forces
Covalent compounds have what type of forces?
1. Dispersion
2. Dipole-dipole
3. H Bonds
Dispersion
Non- polar
Induced dipole formation
Dipole-Dipole
Polar
H Bonds and between
Hydrogen and N,O, F
Physical properties
1. Boiling point
2. Density
3. Melting point
4. Viscosity
5. Solubility
Stronger intermolecular forces have higher
1. Higher boiling point
2. Higher melting point
3. Greater viscosity (related to interaction between layers of molecules)
Dispersion factors are stronger and weaker when?
1. Stronger for higher molar mass (atomic #)
- As the number of electrons increases = more distortion and dispersion
2. Weaker dispersion forces with branching (surface area increased)
All hydrocarbons are
non polar
even though structures look non symmetrical they only have dispersion forces
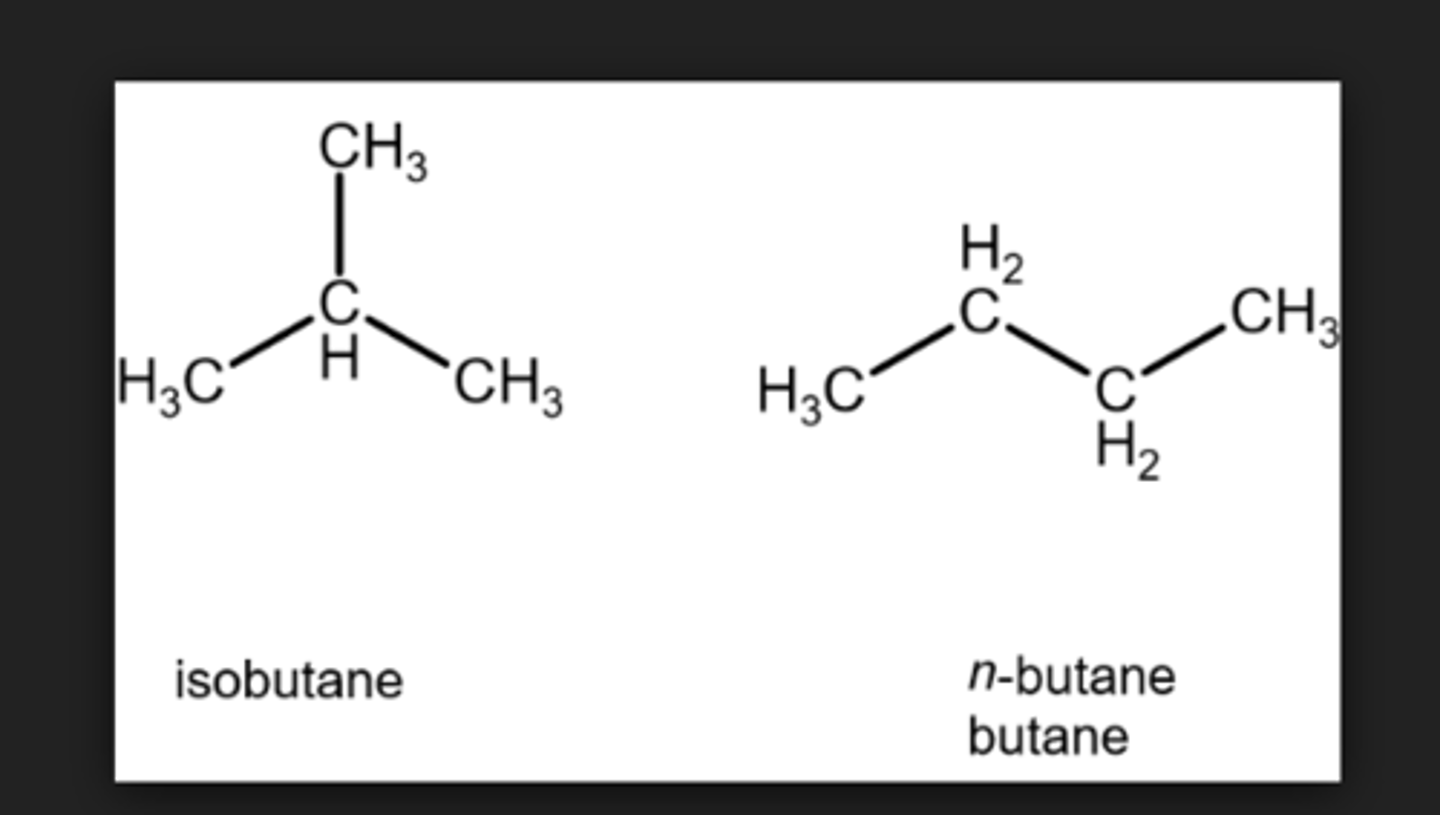
ex. Isobutane C4H10
What has a higher boiling point n-butane or Isobutane?
N-butane because it is not branched
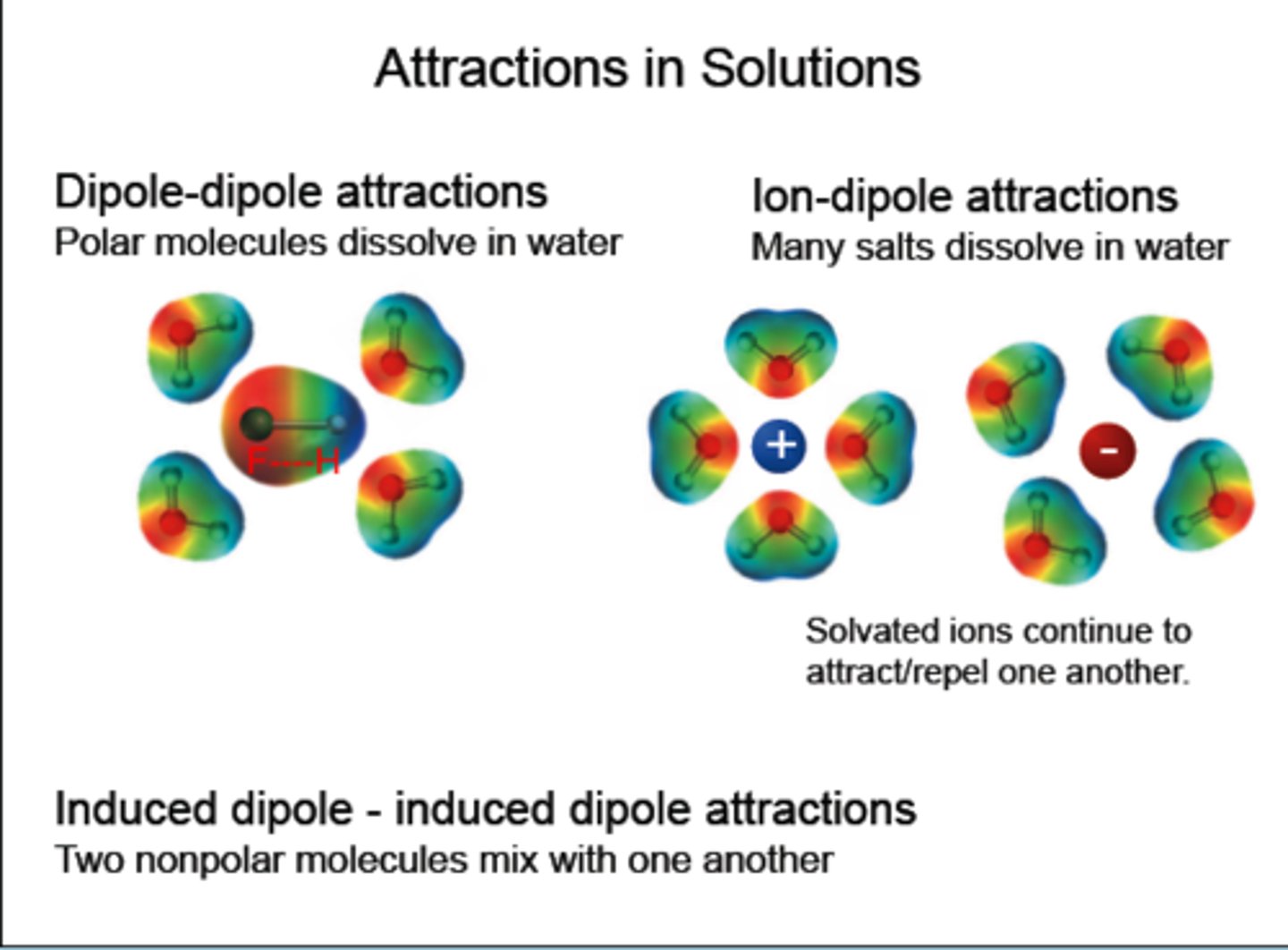
Solubility
"like dissolves like"
A polar compound dissolves ?
A polar compound dissolves another POLAR COMPOUND better than a nonpolar
Benzene (C6H6) dissolves better in H20 or CCl4
CCl4 because it is nonpolar like Benzene
Alcohol are
polar (all have O + H)
HCN
Will not do more than three bonds
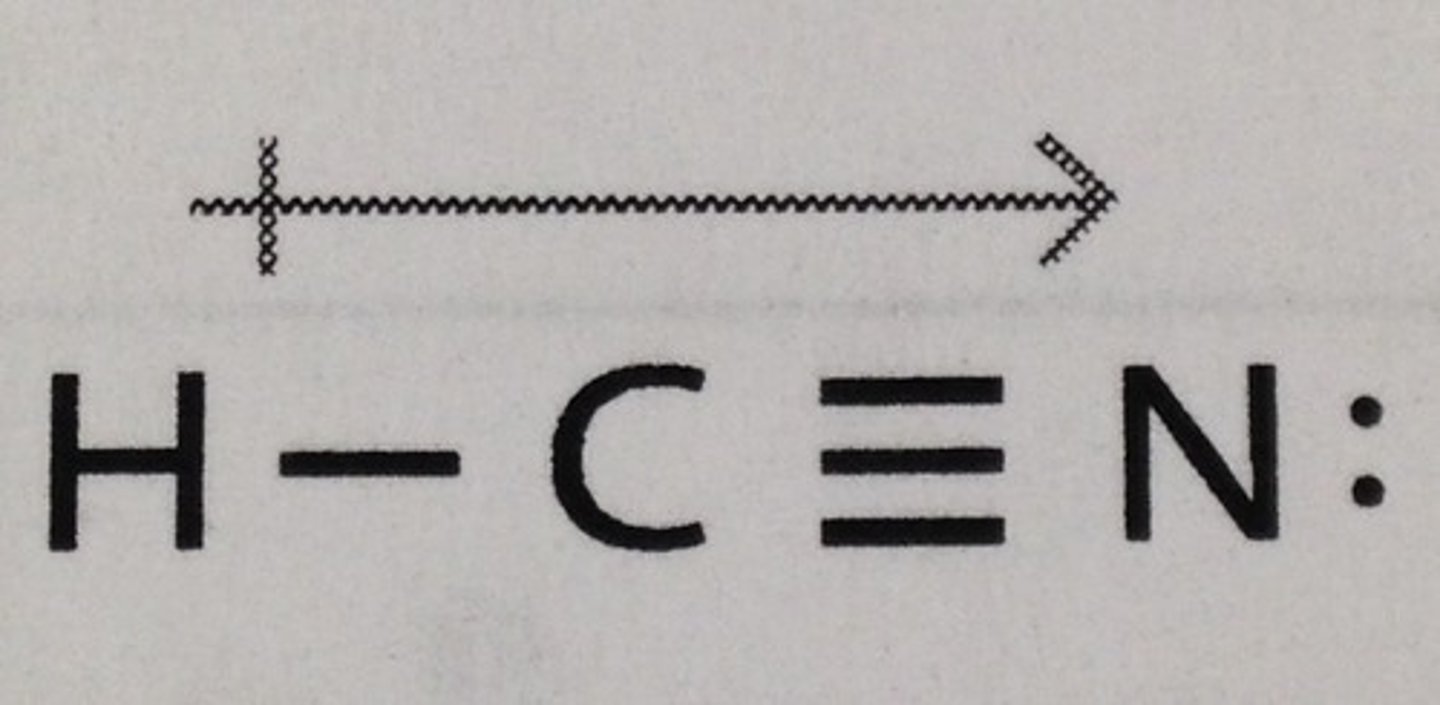
Intermolecular forces of HCN
Dipole - Dipole primarily
No hydrogen bond because hydrogen is bonded to carbon
Will HCN be soluble in water
yes because water is polar
Nuclear charge is greater in He or H?
He > H
He is bond more tightly closer, average distance a little less
Force of attraction in Helium is more than hydrogen
Atomic radius is greater in He and H
Atomic radius is greater in hydrogen than in helium
In the periodic table from left to right the valence shell will be the
same
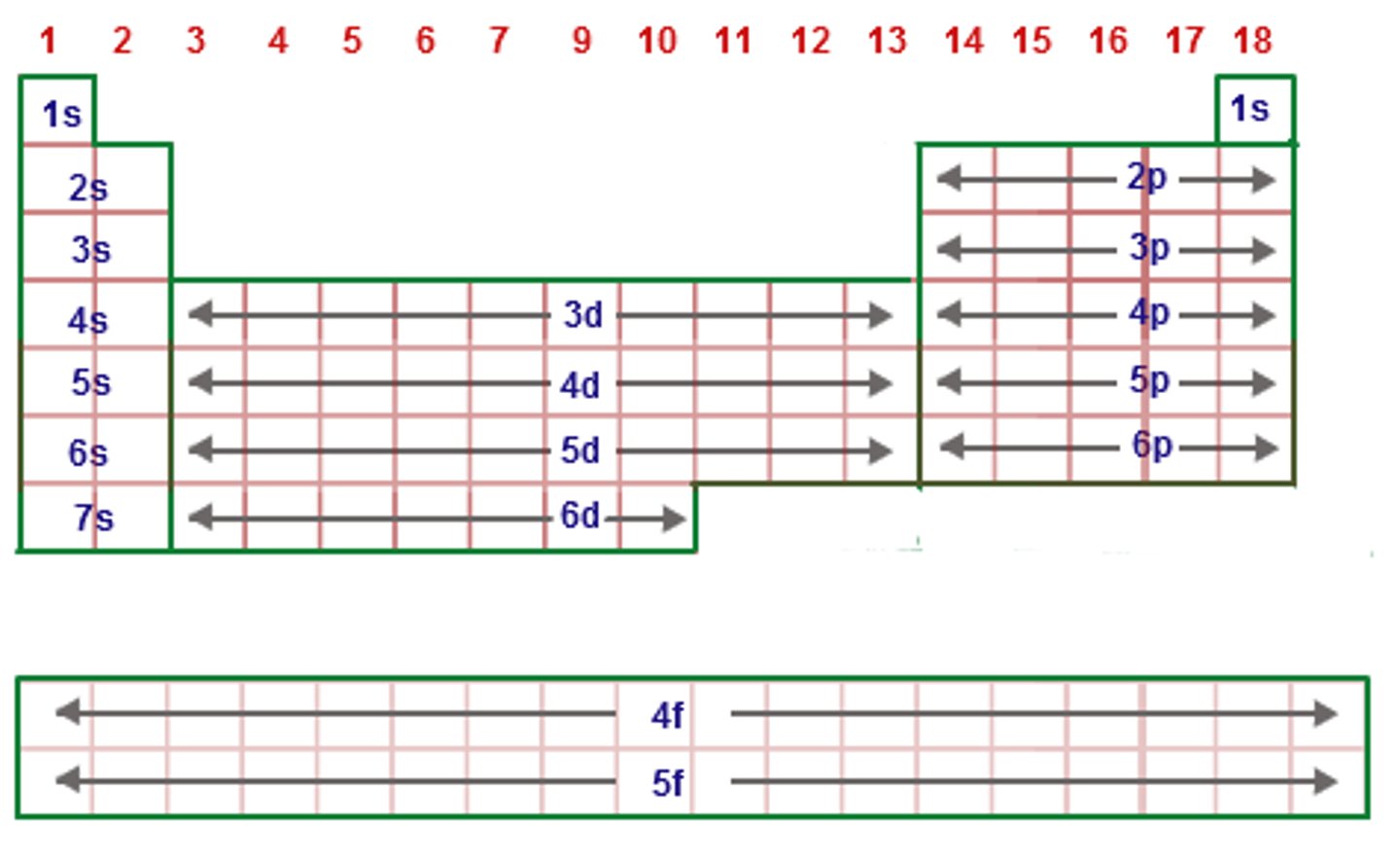
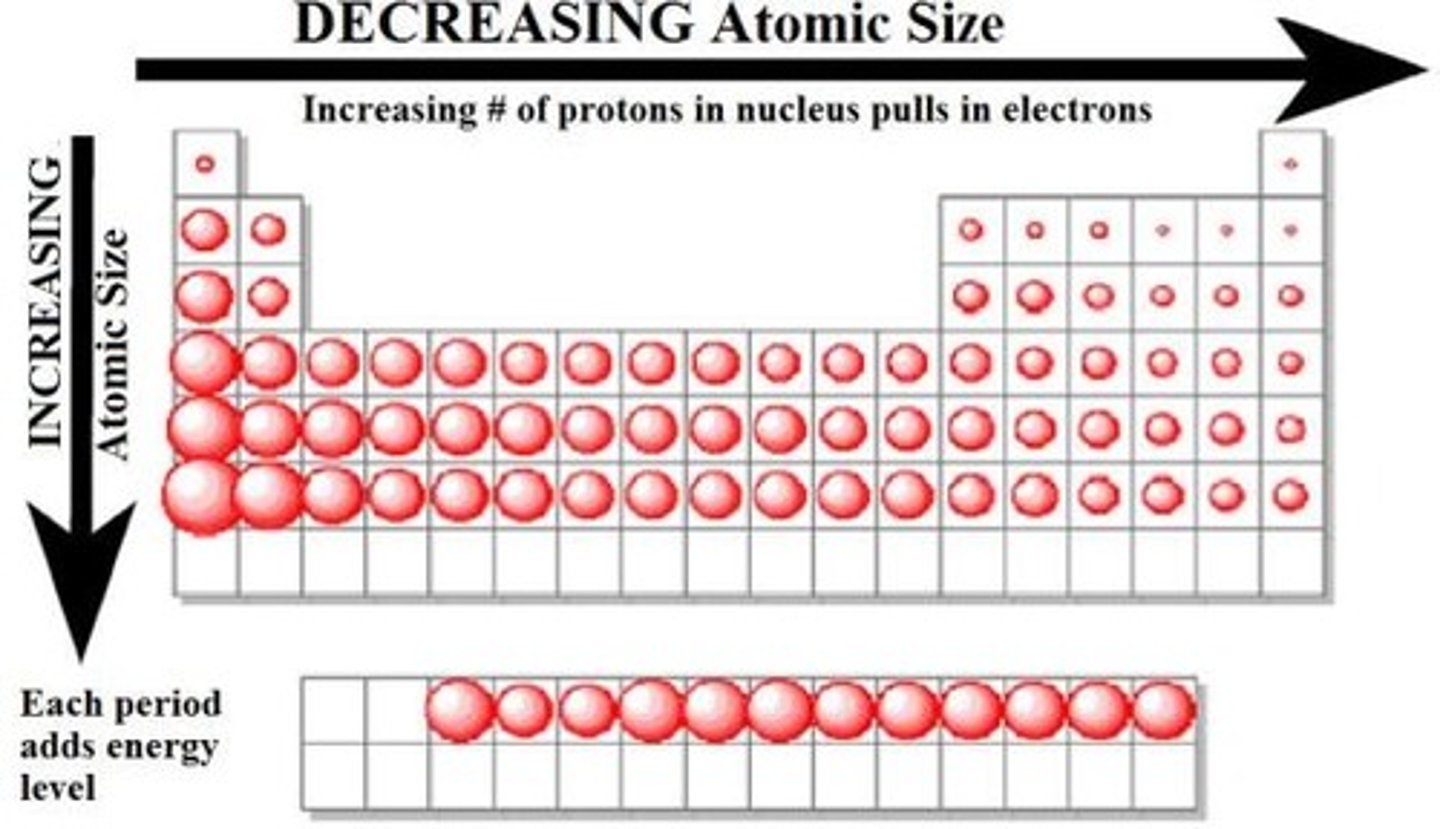
In the periodic table atomic radius
Decreases from left to right (due to increasing nuclear charge)
Increases as you go down the periodic table (increasing electrons) though nuclear charge is increasing valence shell distance is already greater.
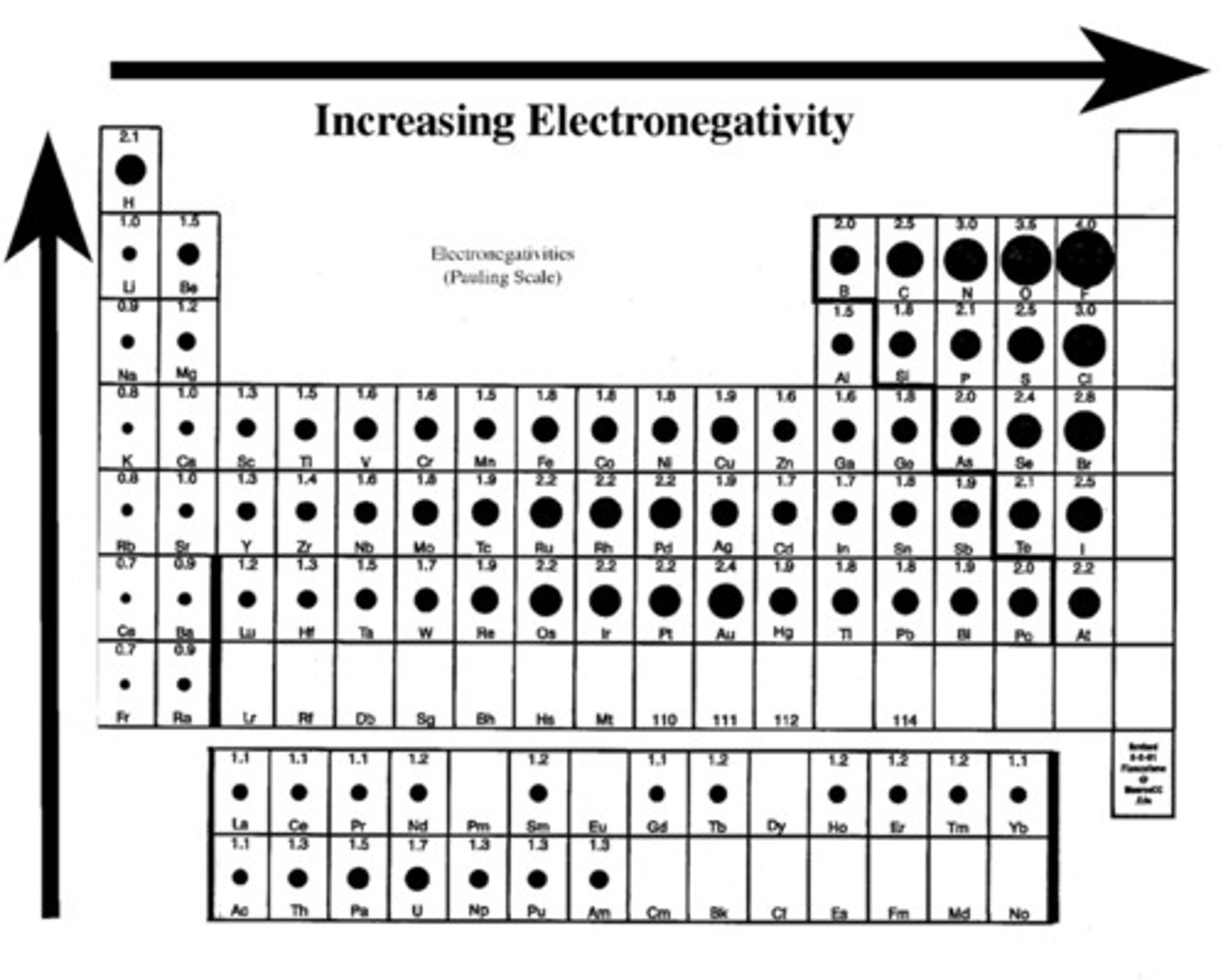
Electronegativity
a chemical property that describes the tendency of an atom to attract a shared pair of electrons
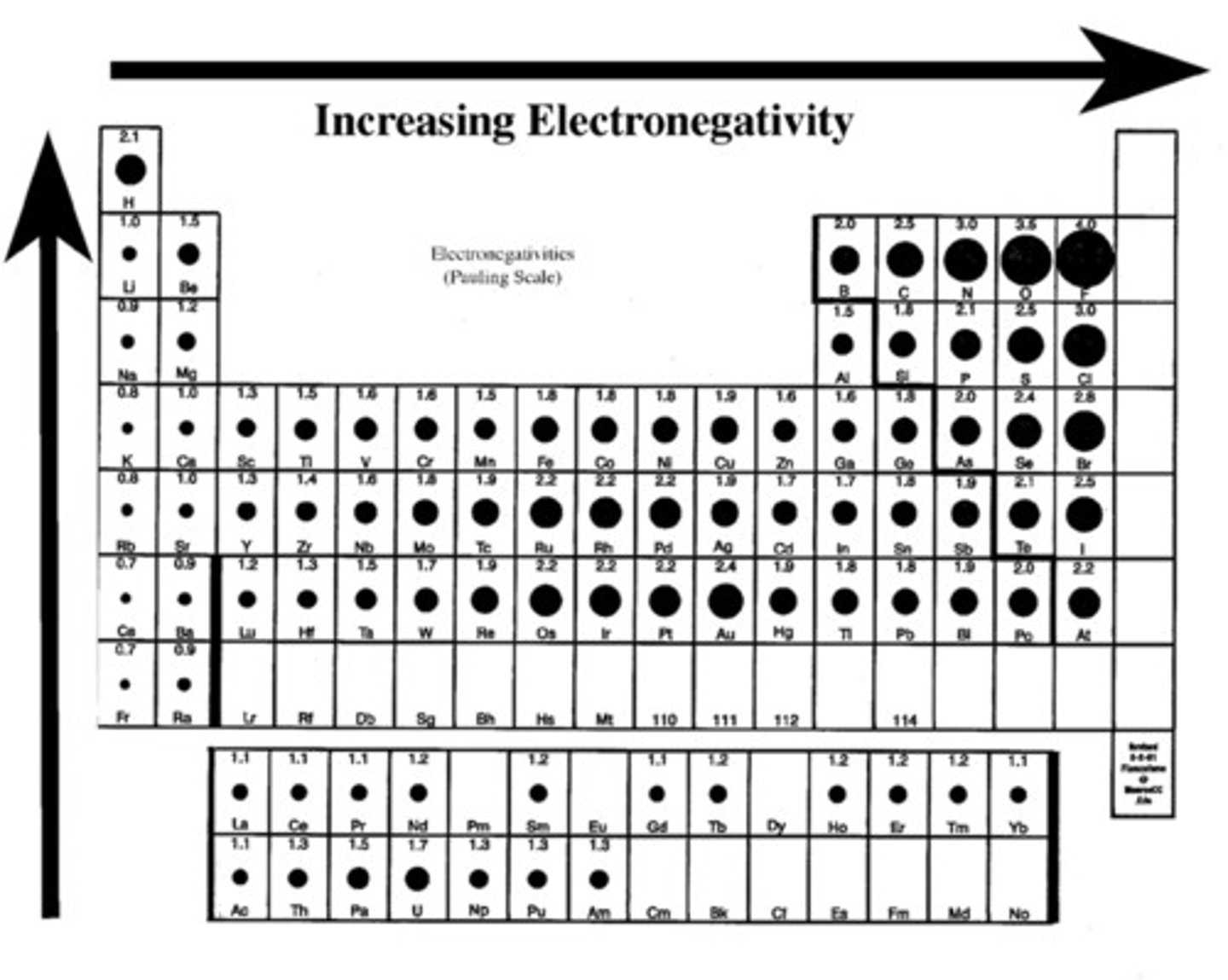
Electronegativity trend in periodic table
1. Electronegativity increases as you go from left to right, attracts more strongly
2. Electronegativity decreases as you go down a period
Ionization energy
The energy required to remove an electron from an atom, an ion, or a molecule
Minimum energy needed to remove a valence electron from a neutal atom
Electronegativity
The relative attraction that an atom has for a pair of shared electrons in a covalent bond
Ionization energy trends in periodic table
Increases from left to right more difficult to remove an electron going towards noble gas configuration
Ionization energy decreases going down table adding more shells
Metallic characteristics in periodic table
Metallic characteristics decreases from left to right
Metallic characteristics increases as you go down (Fr best metal)
Metals make positive charges more easily
Place in increasing order of atomic radius
P,N, S, AL
N > S > P > AL
Go by shell
Ionization energy increasing order
C, Be, Ca, Sr, B, Kr
Sr > Ca > Be > B > C > Kr