01 Experimental Chemistry
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
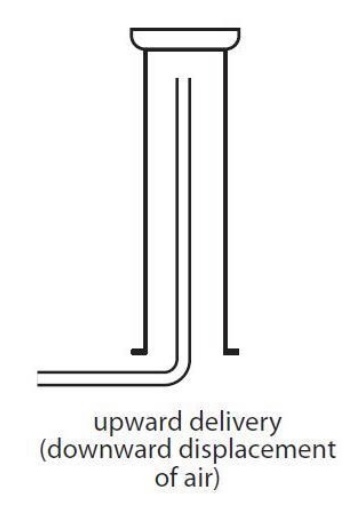
Gas A is soluble in water and less dense than water, what collection method do i use?
upward delivery
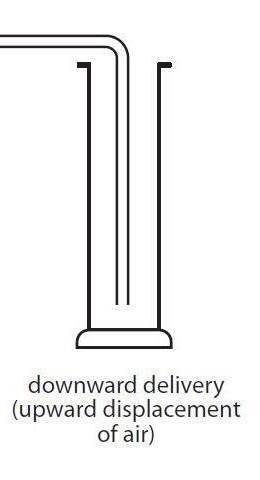
Gas B is soluble in water and denser than water, what collection method do i use?
downward delivery
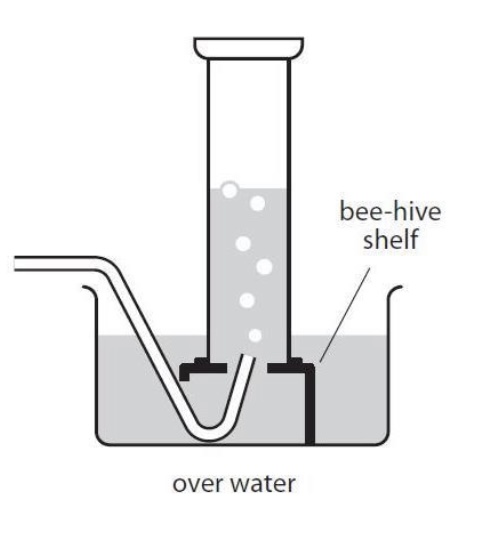
Gas C is insoluble in water, what collection method do i use?
water displacement
How to collect hydrogen?
water displacement with fused calcium chloride
How to collect oxygen?
water displacement with fused calcium chloride
How to collect nitrogen?
Water displacement with fused calcium chloride
How to collect ammonia?
upward delivery with quicklime/calcium oxide
How to collect carbon dioxide?
water displacement with concentrated sulphuric acid
How to collect chlorine?
downward delivery with concentrated sulfuric acid
How to collect nitrogen dioxide?
water displacement with fused calcium chloride
How to collect sulfur dioxide?
downward delivery with concentrated sulfuric acid
How to collect hydrogen chloride?
downward delivery with concentrated sulfuric acid
What is an advantage of using chromatography?
Only a small amount if sample is required for the analysis.
Name the apparatus in a fractional distillation set up.
Thermometer, boiling chips, fractionating column, liebig condenser
How do you know when a liquid in a fractional distillation set up has completely distilled?
The temperature on the thermometer starts to increase above its boiling point.
Describe how a mixture can be separated using filtering.
Filter the mixture througha filter funnel line with filter paper, placed over a conical flask. The insoluble fiber will be lest as residue and collect the filtrate which contains the sugar.
Describe how a mixture can be separated using crystallisation.
Heat the solution to evaporate most of the wster and allow the saturated solution to cool to form crystals. Filter and rinse the residue which is the sugar crystals with a small ampunt of cold distilled water. Dry the crystals between sheets of filter paper.
How does a condenser allow alchohol to be collected as a liquid in a distillation set up?
It provides a coller surfacr that causes the hot vapour to lose energy and condense.