Isomers CHEM 352
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
isomers
different structure, same molecular formula
stereoisomers
different orientations, same connectivity, similar properties
(differ in spatial arrangement of atoms, rather than order of atomic connectivity)
constitutional isomers
same molecular formula, different connectivity
chirality
molecule cannot be superimposed on its mirror-image
enantiomer
non-superimposable mirror-image molecules, similar physical properties
all chiral centers are inverted
diastereomer
non-superimposable, non-mirror-image
cis/trans
only some chiral centers are inverted
optical activity
ability to rotate plane polarized light
chiral center
sp³ hybridized → tetrahedral
4 electron groups, rankable by atomic number
Finding R or S
Prioritize groups by higher atomic number
Put “4” in back (dash)
Count 1,2,3 R (clockwise) and S (counter clockwise)
Fisher Projections
most useful for drawing molecules with multiple chirality centers such as sugars and can quickly access stereoisomeric relationships
skeleton with a bow tie
horizonal lines (coming out of page) and vertical (back into page)
E/Z
used for alkenes
each sp² hybridized C has two diff groups
“z” same side
occurs cus double C=C bonds can’t rotate

cis/trans
cis (same) vs trans (opposite)
used for alkenes
each sp² has only 1 H and a diff. group
used when there are common substituents on adj. C’s
plane of symmetry
if a molecule has this, it is achiral
%ee
(observed/specific) * 100% = more R/S than R/S, depending on the sign matching
observed = mixture specific rotation
specific = of either the R or S compound
will never be more than 100%
the specific rotation of R and S will be the same number but different signs
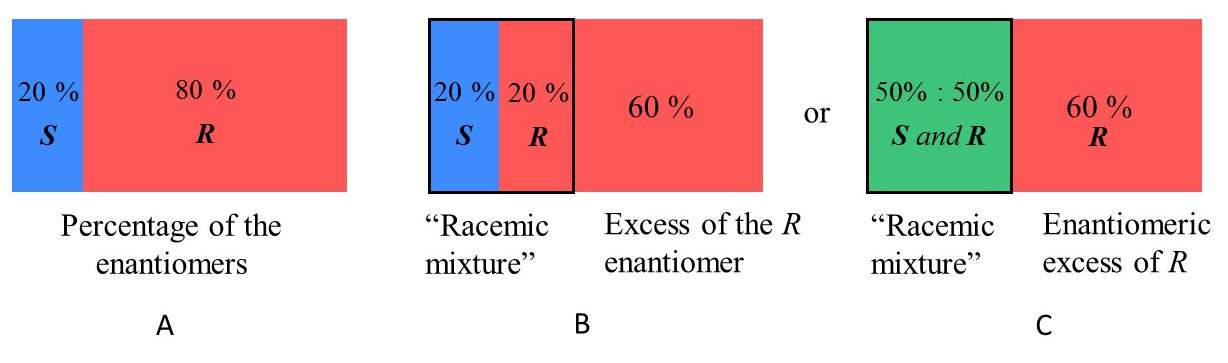
racemic mixture
50:50 mixture of two enantiomers
cancel each other out and the mixture is optically inactive