Mid term 3 orgo 2
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Isolectric point definition and how to find it
Average the two pKa values
Vertical Transition points
Located N
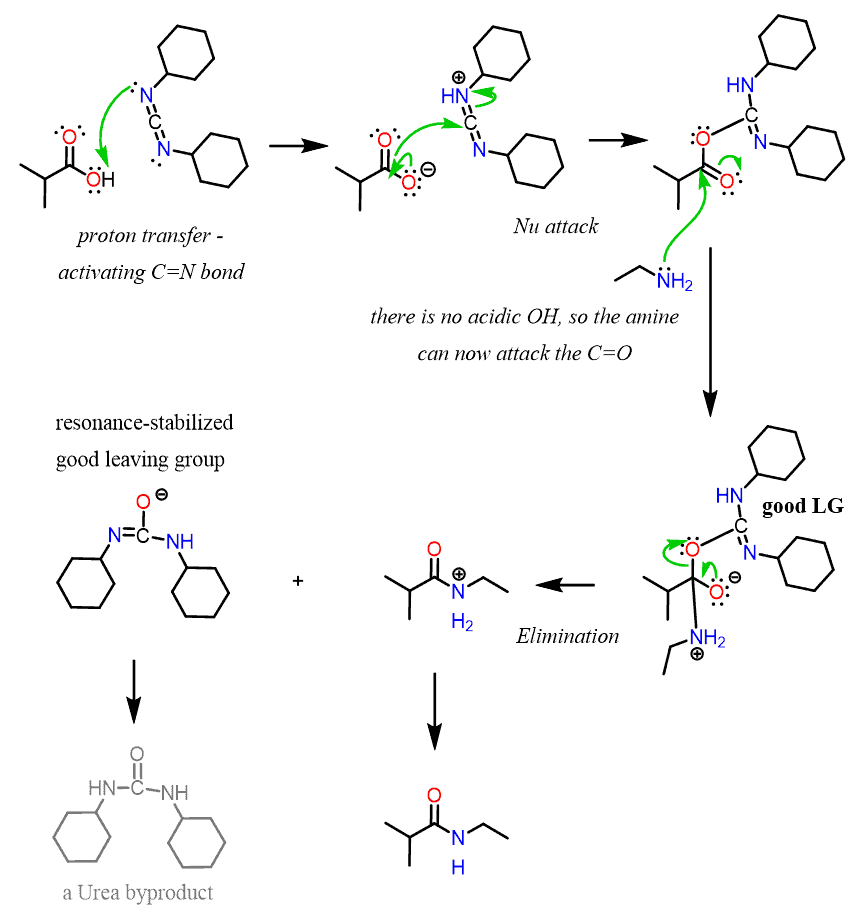
the hydrolysis of peptide bonds
6 M HCl, 110 °C, 24 hrs.
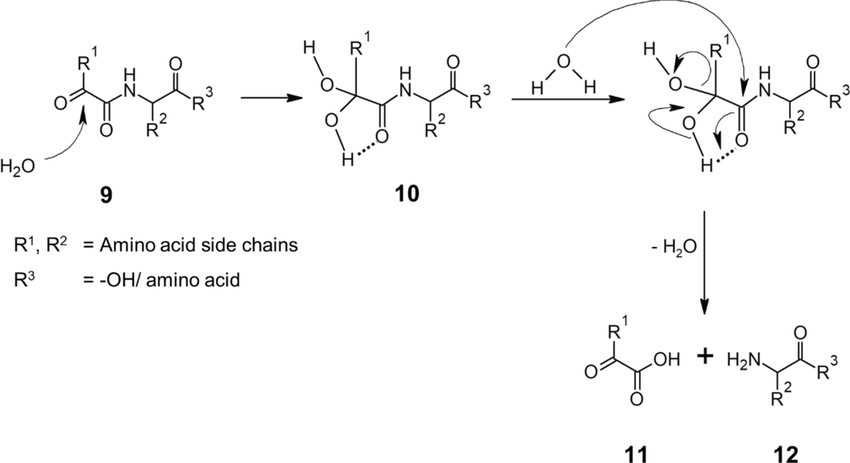
electron-pushing mechanism for the DCC method of amide formation from DCC
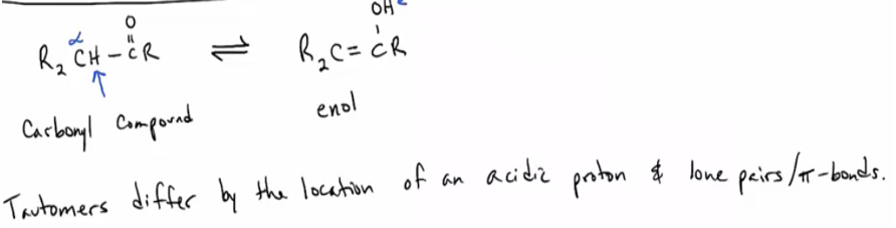
Define and draw compounds that are related as tautomers
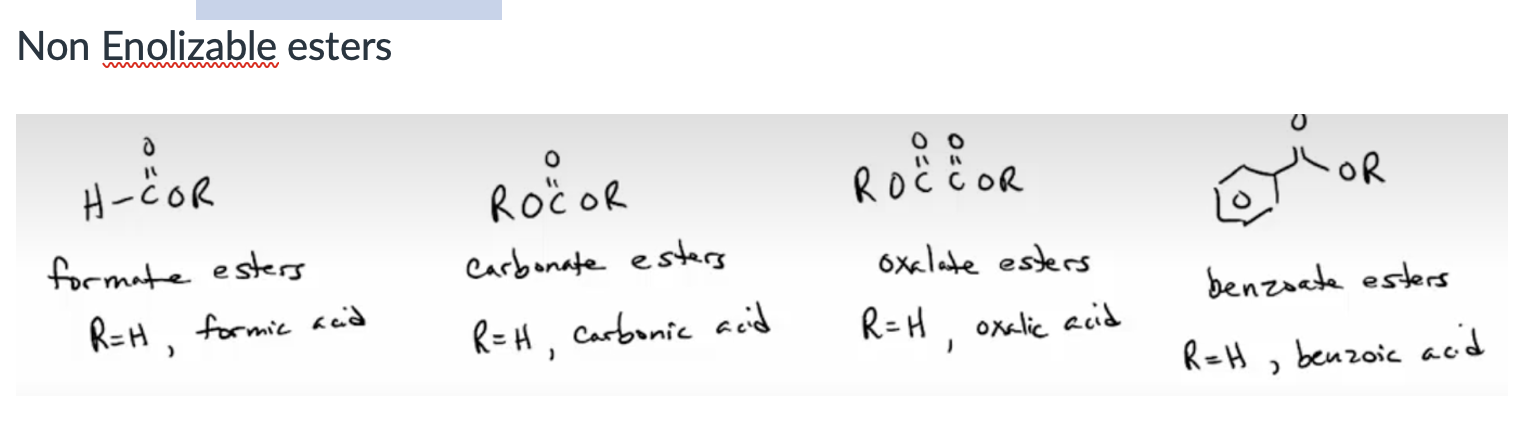
Form ester enolates from the Bronsted-Lowry acid/base reaction of esters with lithium dialkylamides. (You often need a stronger, N- base if there aren’t two C=O’s beta to each other.)
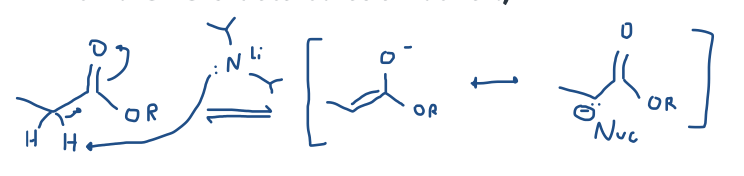
Rank acidities of various carbonyl-containing functional groups and their approximate pKa. See Table 22.1.
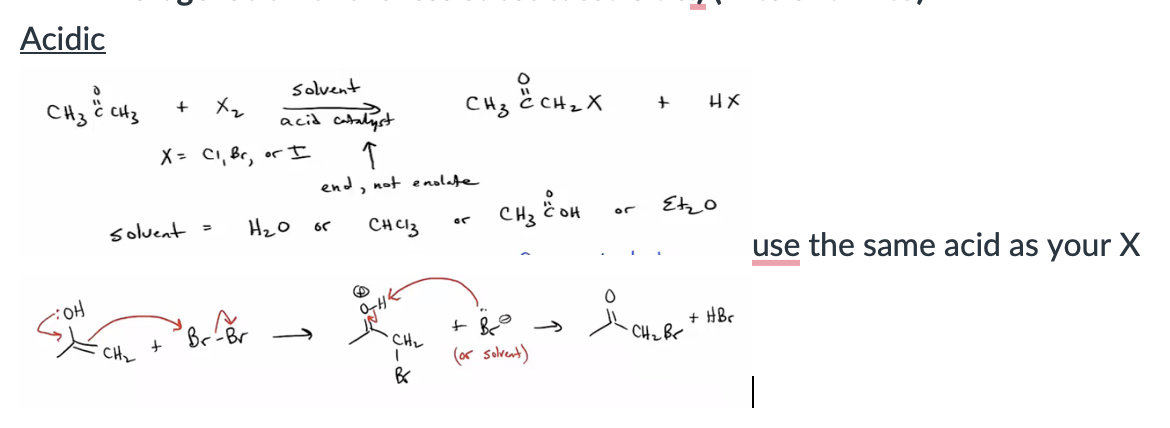
Provide products and mechanisms for halogenation of aldehydes and ketones under acidic conditions
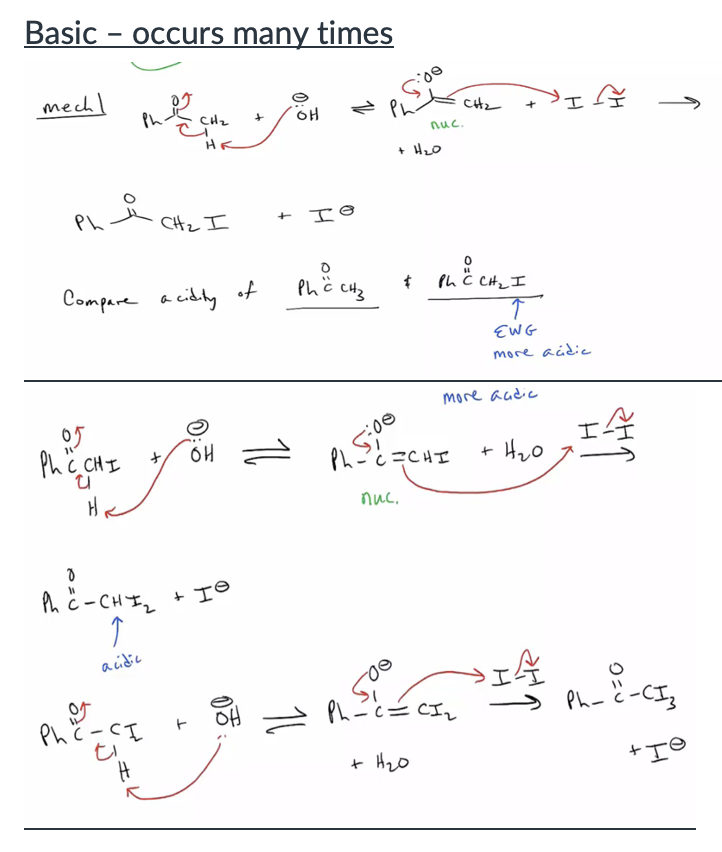
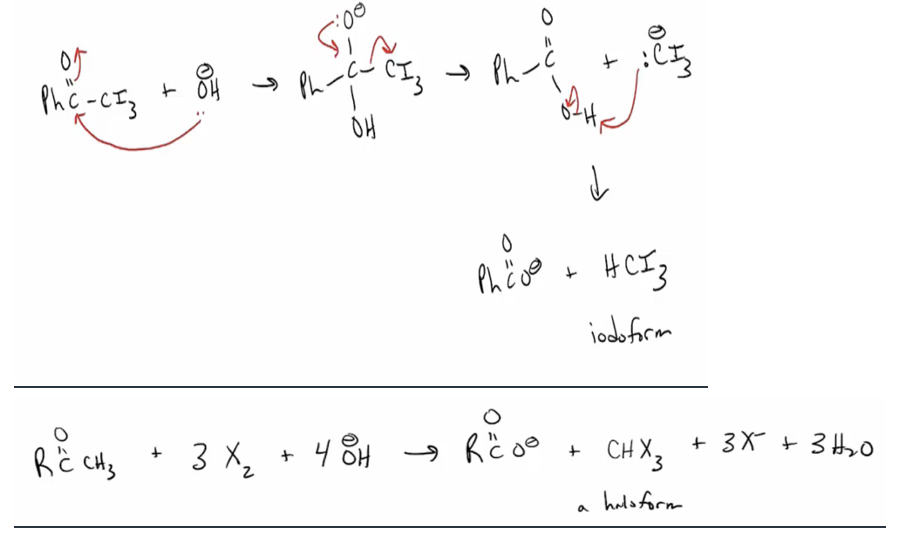
Provide products and mechanisms for halogenation of aldehydes and ketones under basic conditions
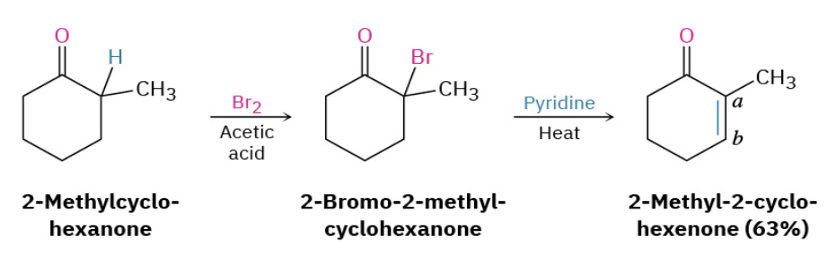
Provide products and a mechanism for the dehydrobromination of bromoketones
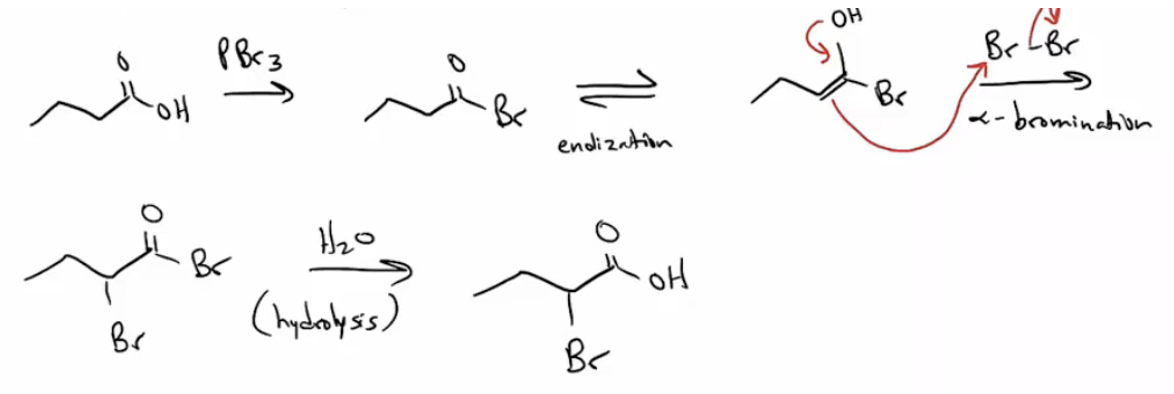
Provide products, mechanism, or missing reagents for the Hell-Vollhard-Zelinskii reaction
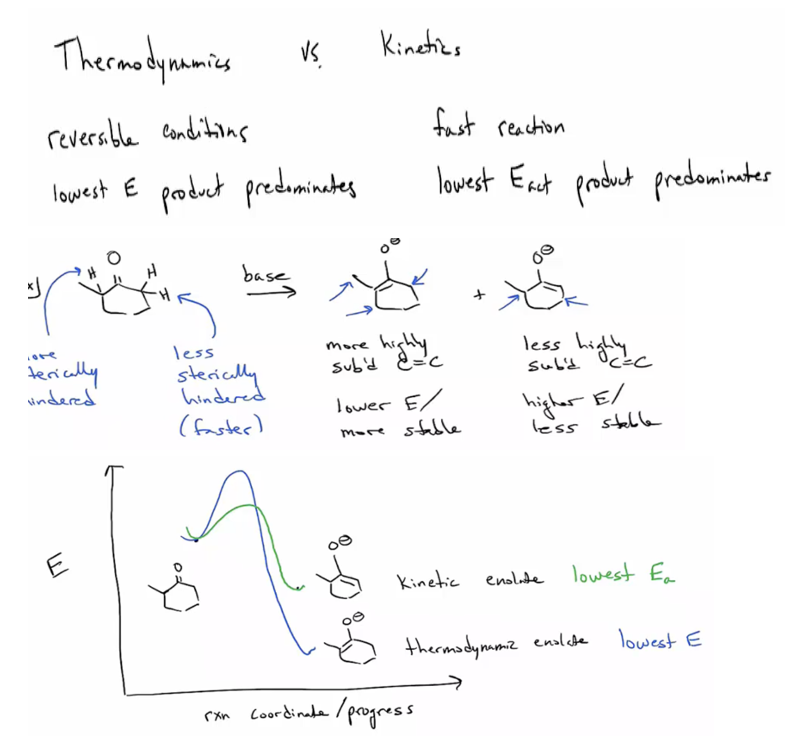
Distinguish between kinetic and thermodynamic enolates and know the conditions to form each. (Strong, irreversible base, such as LDA, and low temp. favor kinetic enolate formation. Weak, reversible base, such as OH- or an amine, and high temp. favor thermodynamic enolate.
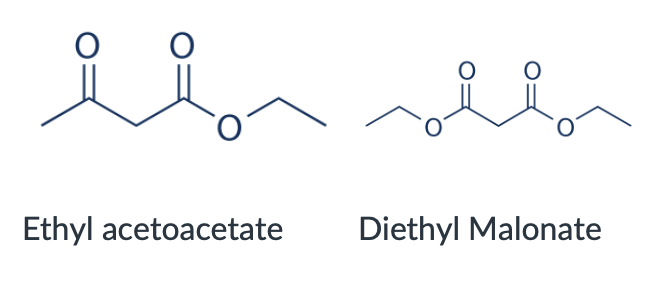
Provide the structures of ethyl acetoacetate and diethyl malonate.
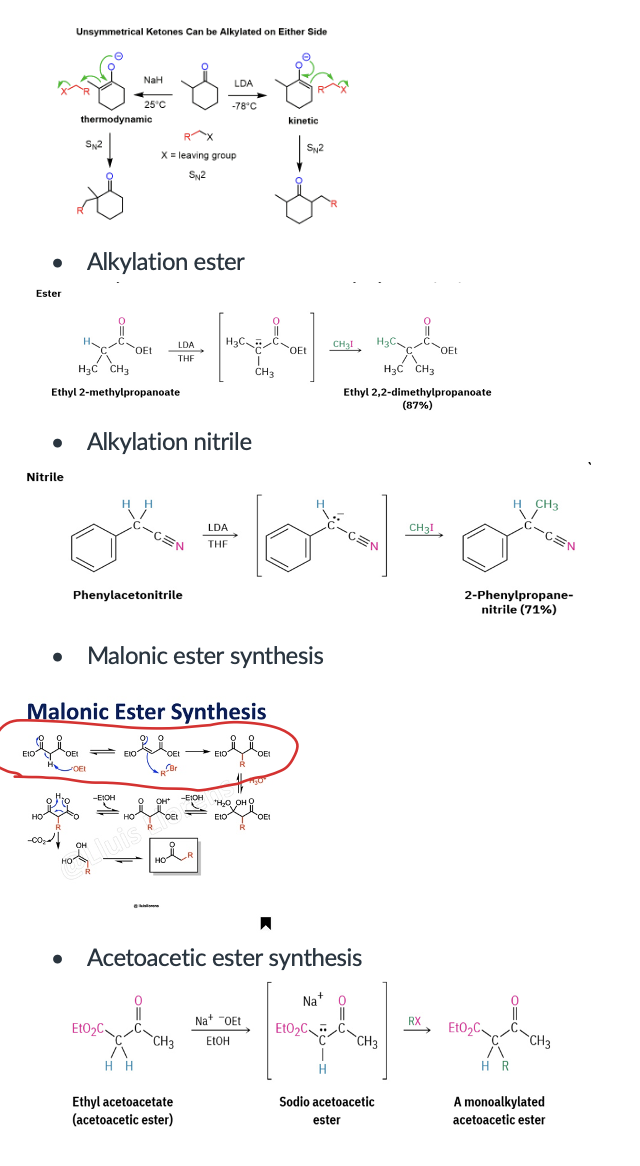
Provide products and mechanisms for the various alkylation reactions, including alkylation of a ketone, ester, nitrile, the malonic ester synthesis, and the acetoaceitc ester synthesis
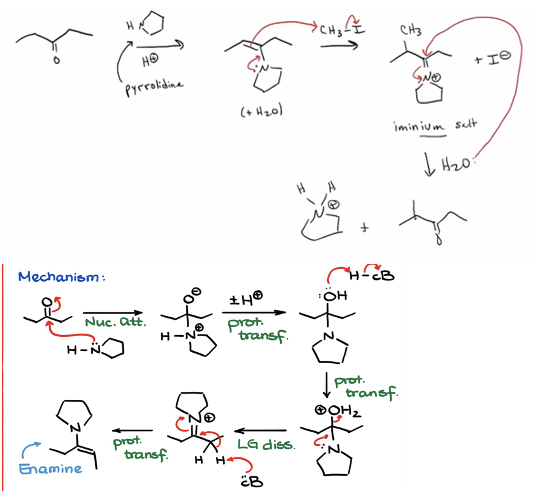
Provide products and mechanisms for converting an aldehyde or ketone to its enamine, alkylating the enamine, and hydrolyzing the resulting iminium salt back to an aldehyde or ketone.
Electrophiles that can be used
· Methyl halide
· Allylic or benzylic halide
· Alpha halo ethers
· Alpha halo ester
· Acid chloride
· Acid anhydride
Provide products and mechanism for the malonic ester synthesis. Followed by ester hydrolysis and decarboxylation, the reaction is used to synthesize carboxylic acids.
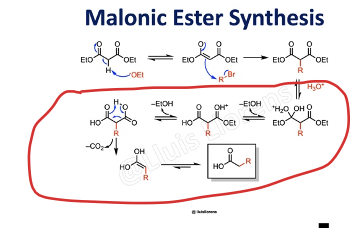
Provide products and mechanisms for the decarboxylation of diesters. The product will be a carboxylic acid (and CO2 and two alcohols) (This represents a continuation of the malonic ester synthesis.)
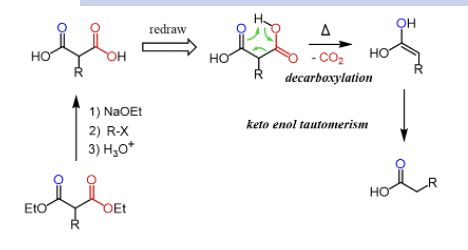
Provide a full electron-pushing mechanism for the decarboxylation of carboxylic acids that have a C=O on the carbon.
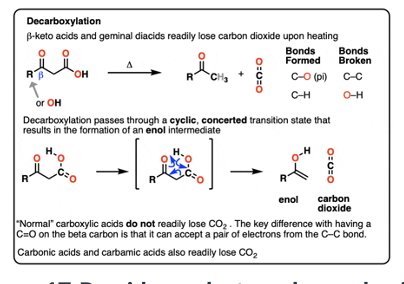
Provide products and a mechanism (SN2) for the reaction of an enolate with an alkyl halide, such as the acetoacetic ester synthesis. Followed by ester hydrolysis and decarboxylation, the reaction is used to synthesize methyl ketones
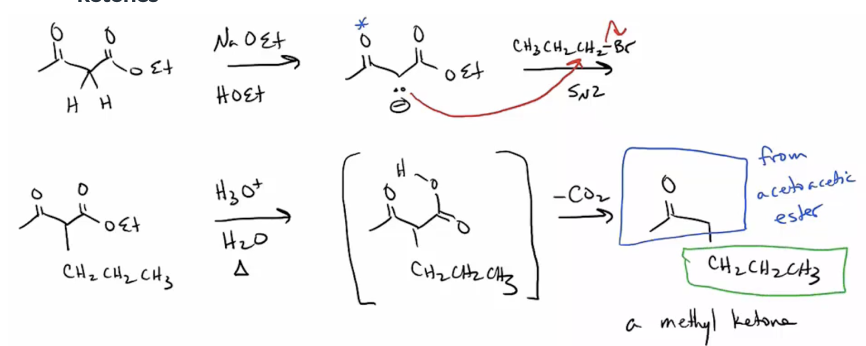
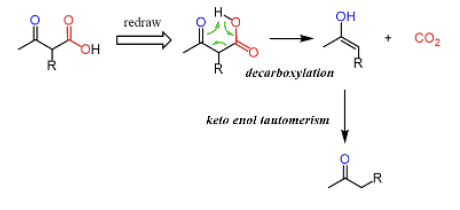
Provide products and mechanism for the decarboxylation of keto esters. The product will be a ketone (and CO2.) (This represents a continuation of the acetoacetic ester synthesis.)
Provide products and mechanism for the aldol and mixed aldol reaction (to give a hydroxycarbonyl compound) and condensation (to give an unsaturated carbonyl compound.) (23.2, 23.3, 23.5, and 23.6)
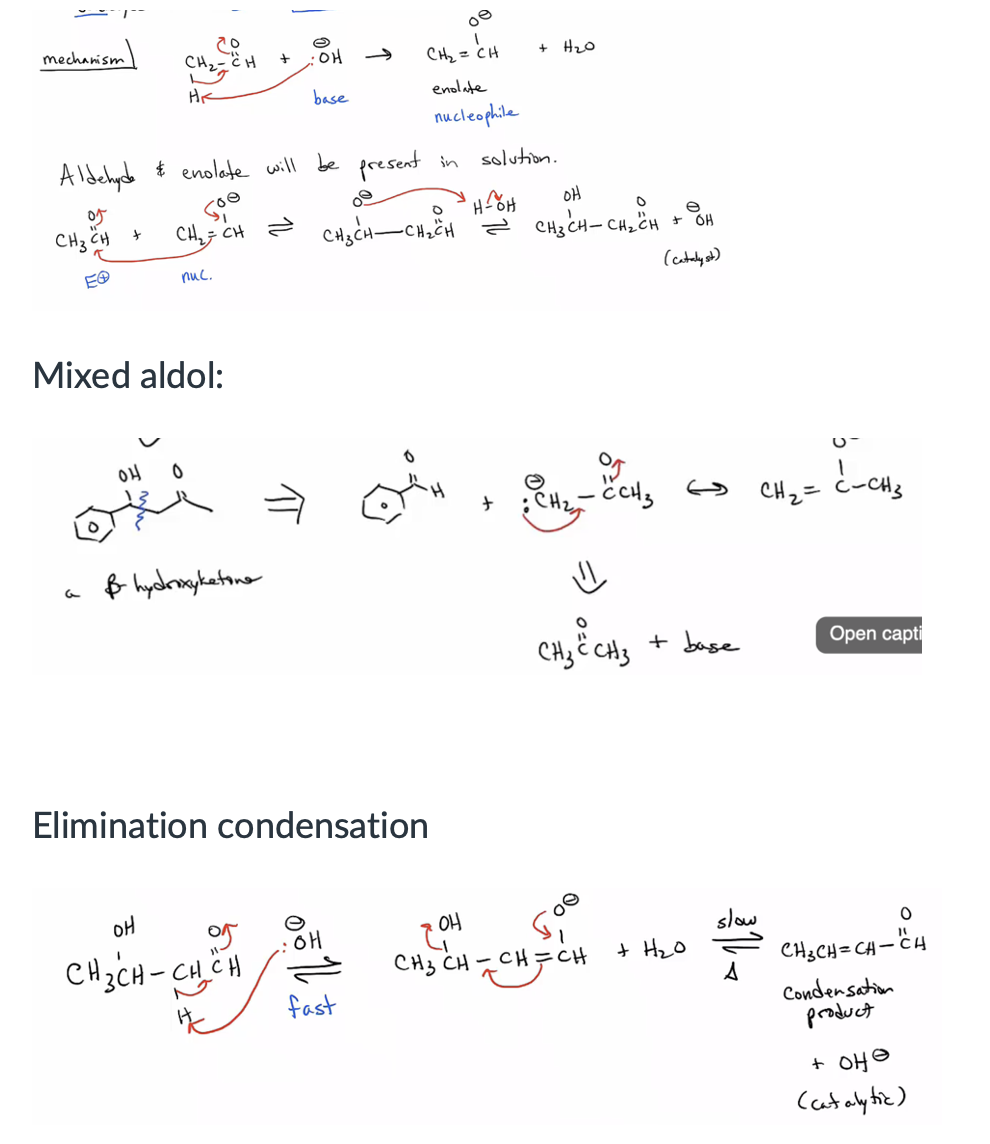
Recognize and use the different conditions for self-condensation of a carbonyl compound with its own enolate versus forming the enolate and using it to react with another electrophile. (23.2)
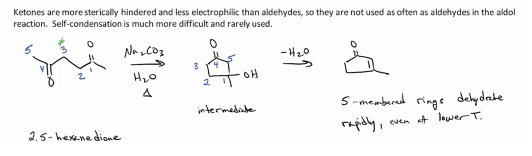
Ketones used less commonly, and usually used with its self to form ring. They do not readily undergo self condensation
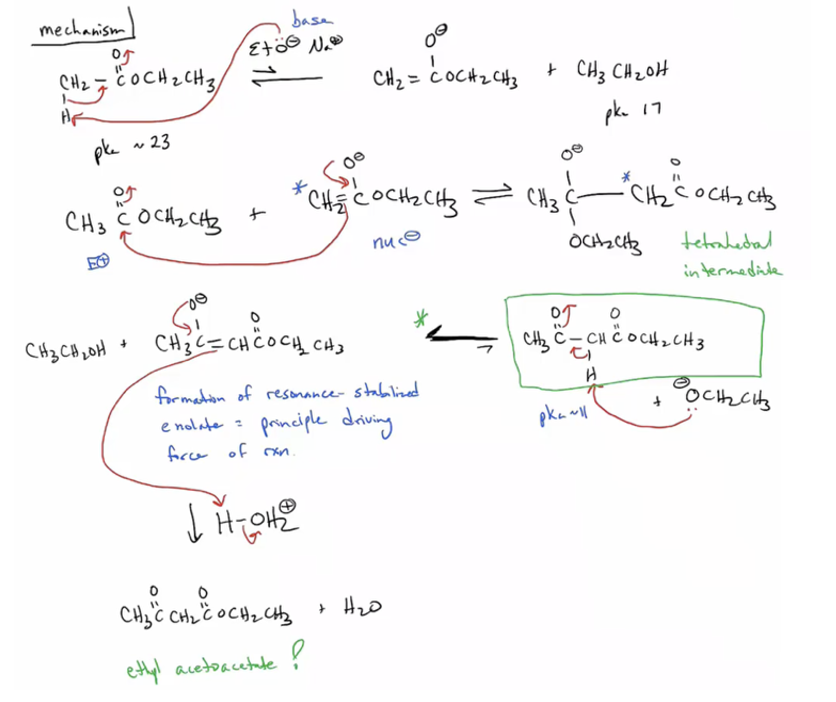
Provide products and mechanism for the Claisen condensation. (One ester molecule forms enolate and then the enolate reacts with another identical ester molecule forming a keto ester.)
Provide products and mechanism for the mixed Claisen condensation. (Use two different ester molecules for the Claisen condensation. The reaction is only useful if one of the esters has an hydrogen and can form an enolate while the other ester cannot form an enolate and thus can only behave as an electrophile.)
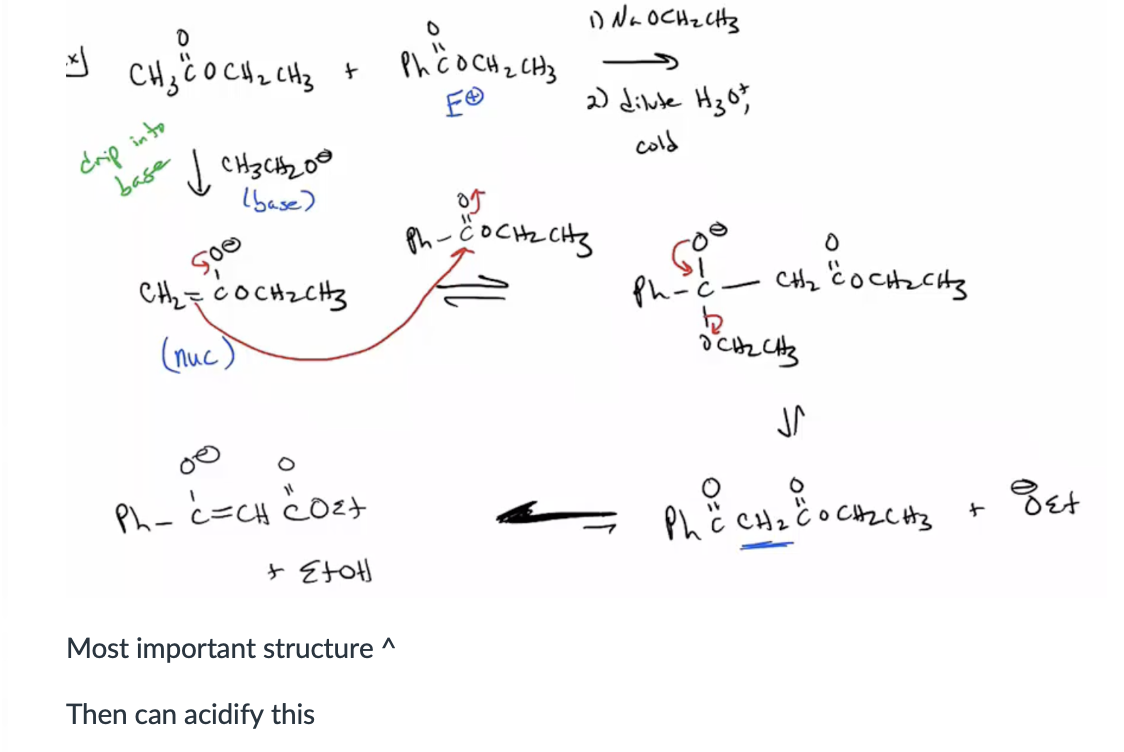
Provide products and mechanism for the Dieckmann condensation. (Simply an intramolecular Claisen condensation. You need two esters in the same molecule to get a cyclic keto ester. 5-, and 6- membered rings are formed easily. Sometimes 4- and 7-membered rings are formed, but not usually.)
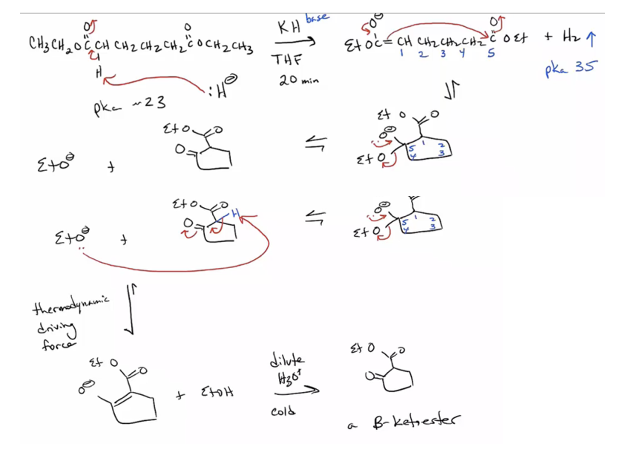
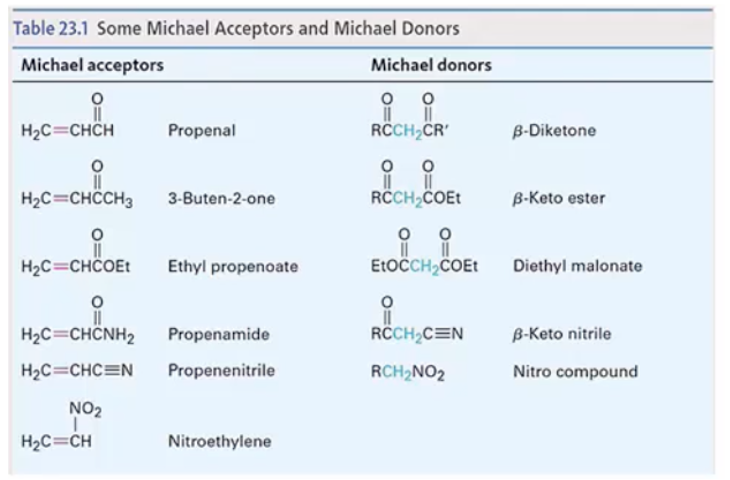
Provide products and mechanism for the Michael Reaction. (Product is often a 1,5-dicarbonyl compound.) Stabilized enolates, such as those of a dicarbonyl compound, are required. (Enolates formed from monoketones won’t do Michael additions.) Anions of Michael donors listed in Table 23.1 will do conjugate additions. (See also 19.13.)
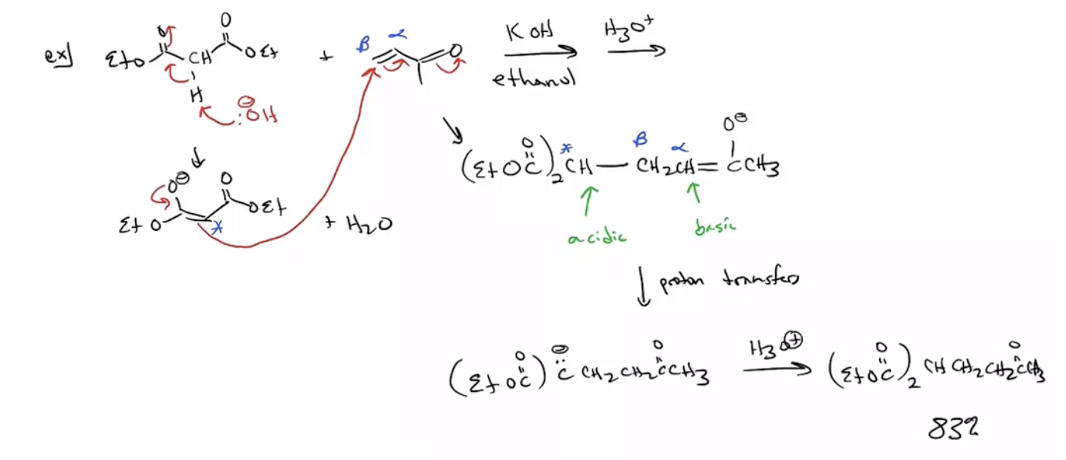
Provide products and a mechanism for the Stork reaction, which involves a conjugate addition of an enamine to a Michael-type acceptor. (The product of the addition is typically hydrolyzed to yield a 1,5-dicarbonyl compound.)
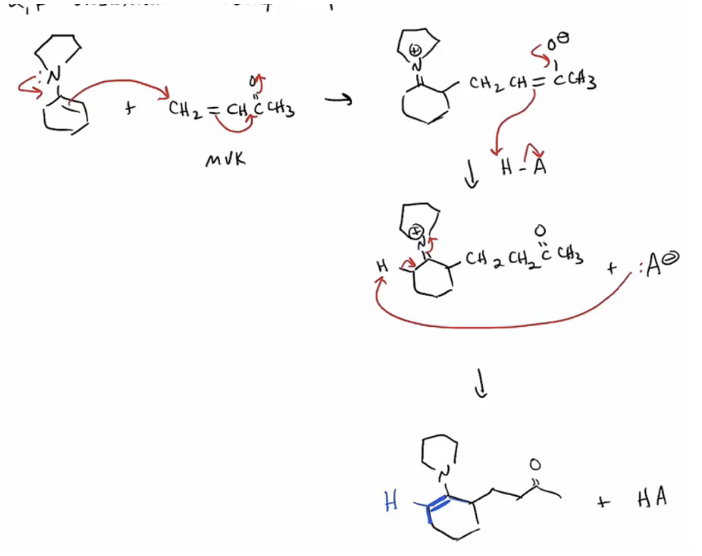
1. Ketone converted to enamine (acid catalyzed)
2. Enamine added to alpha beta unsat
3. Hydrolized to return to ketone
Provide products and mechanism for the Robinson annulation (a Michael addition followed by an intramolecular aldol.)
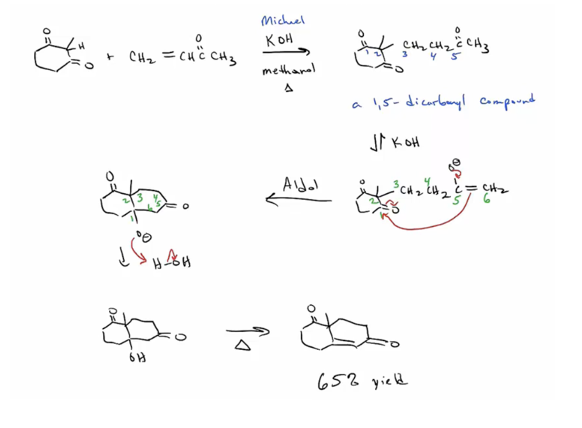
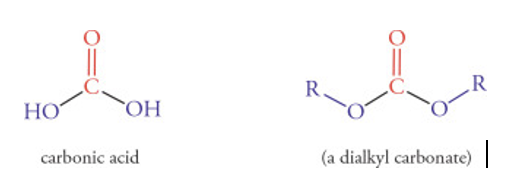
11. Product is B keto ester if a dialkyl carbonate is the ester and the product is a B diketone if a “normal” ester is used.