Bio 1113 Exam 1 OSU
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
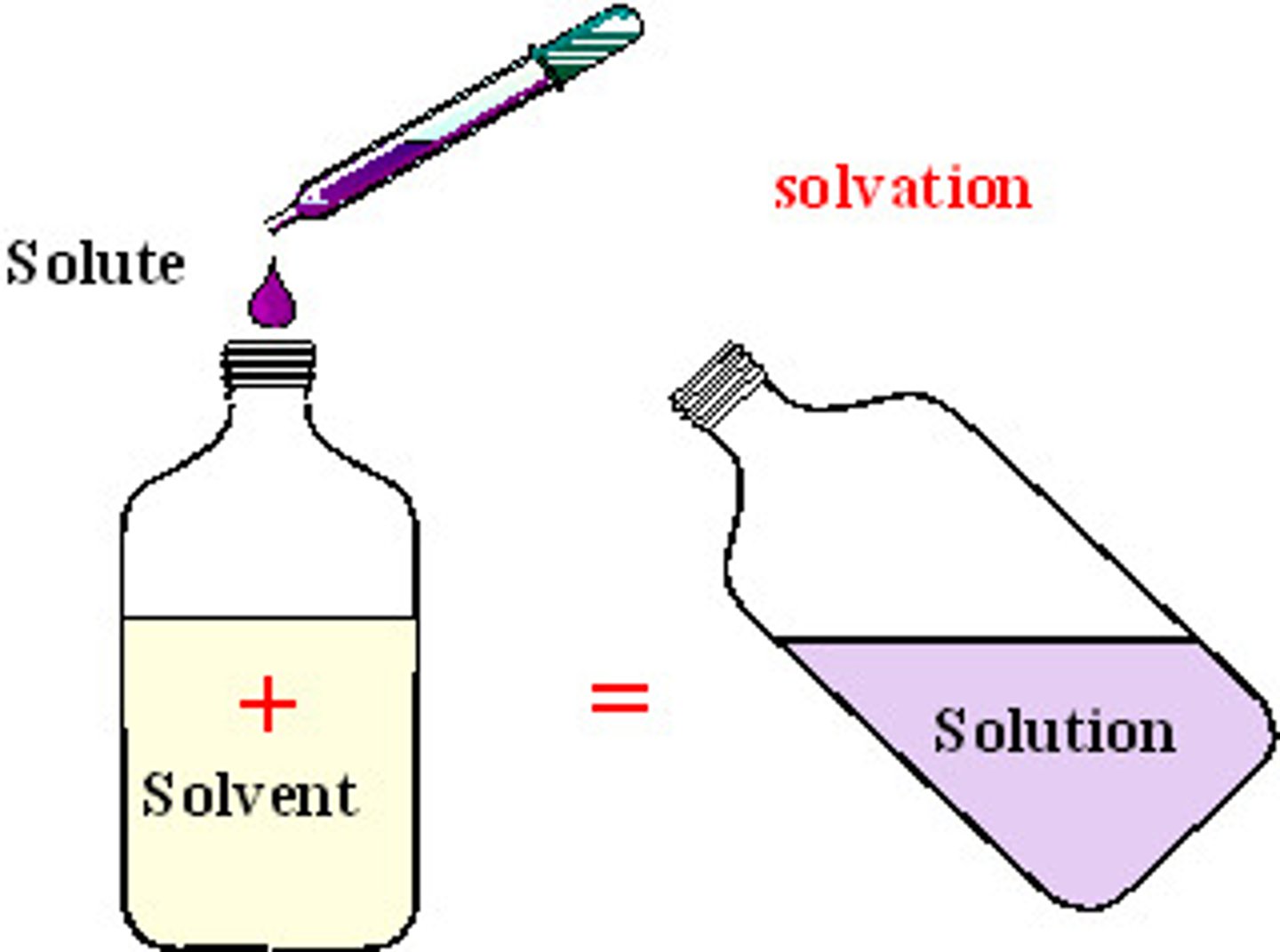
Solvent
Agent for dissolving. Water is an excellent solvent because due to the polarity of the water molecule, it is able to form hydrogen bonds with ions and polarmolecules.
Solute
What is being dissolved.
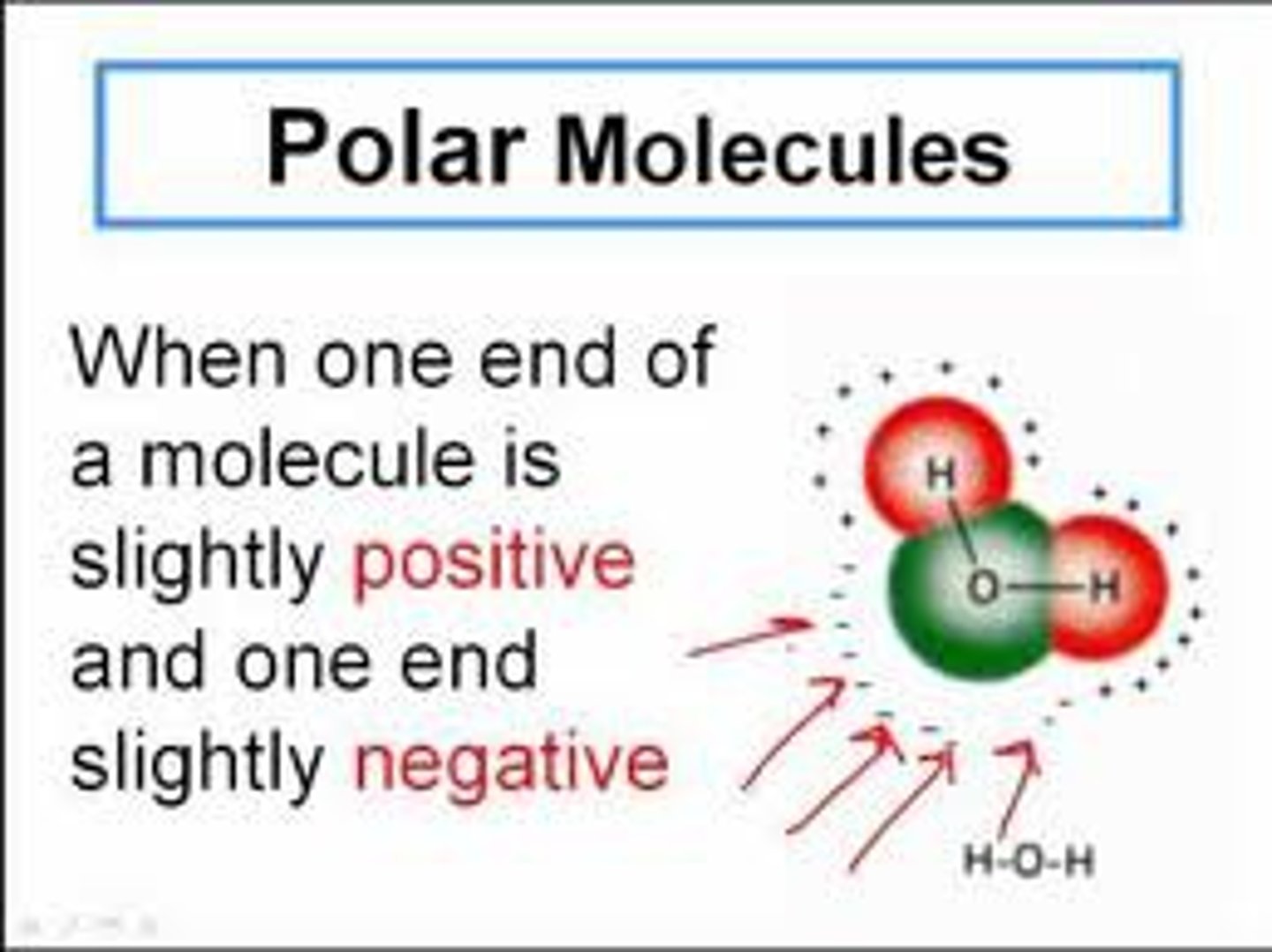
Is water polar?
Yes. It carries a partial positive charge on one side and a negative charge on the other.
Hydrophilic
"water loving." Substances that interact with water. ex. ions and polar molecules because of the interactions with waters partial charges.
Hydrophobic
substances that do not interact with water. Nonpolar molecules do not interact with water and thus don't dissolve in aqueous solutions.
Ionic Bond
is the complete transfer of valence electron(s) between atoms.
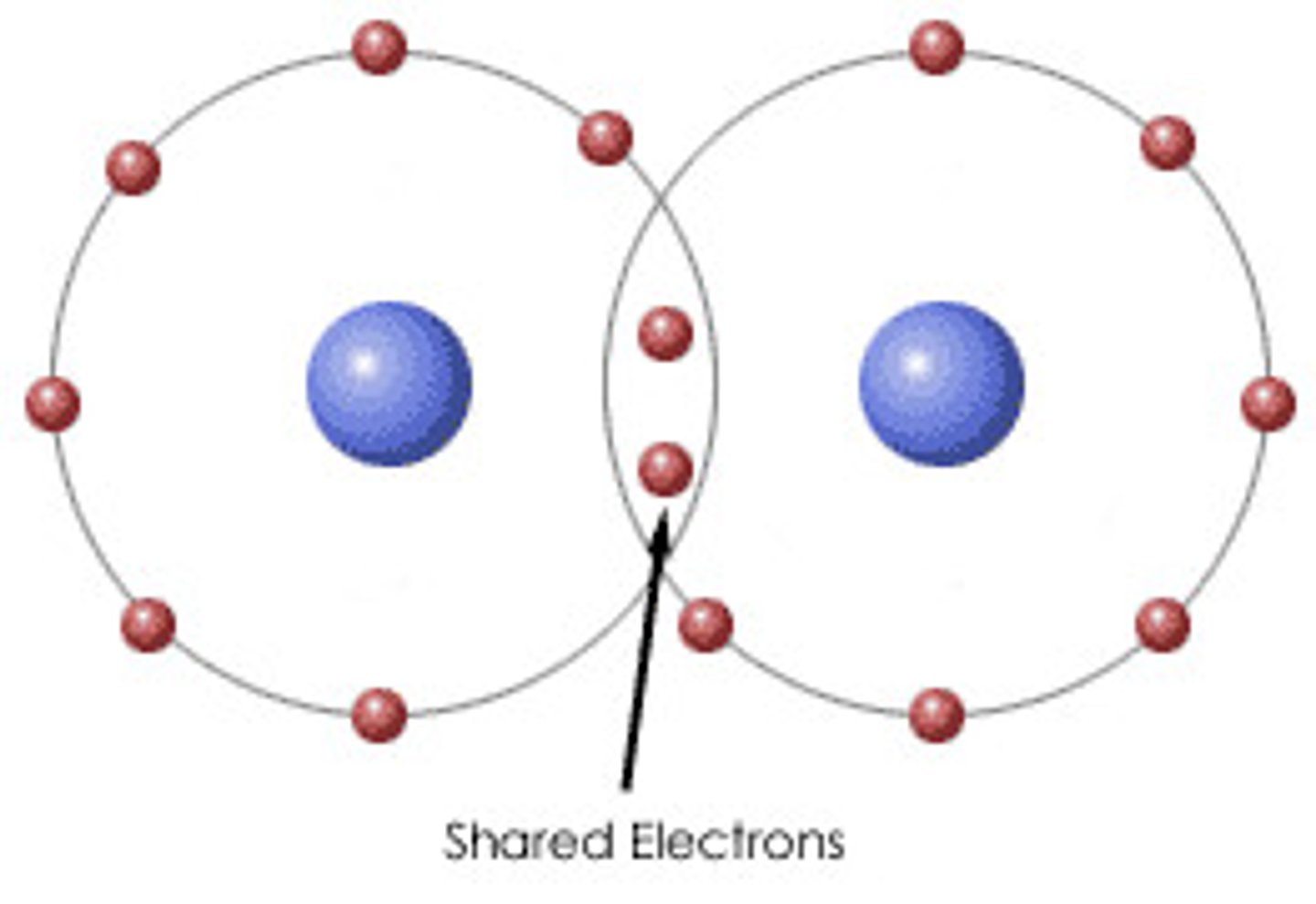
Covalent Bond
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell.
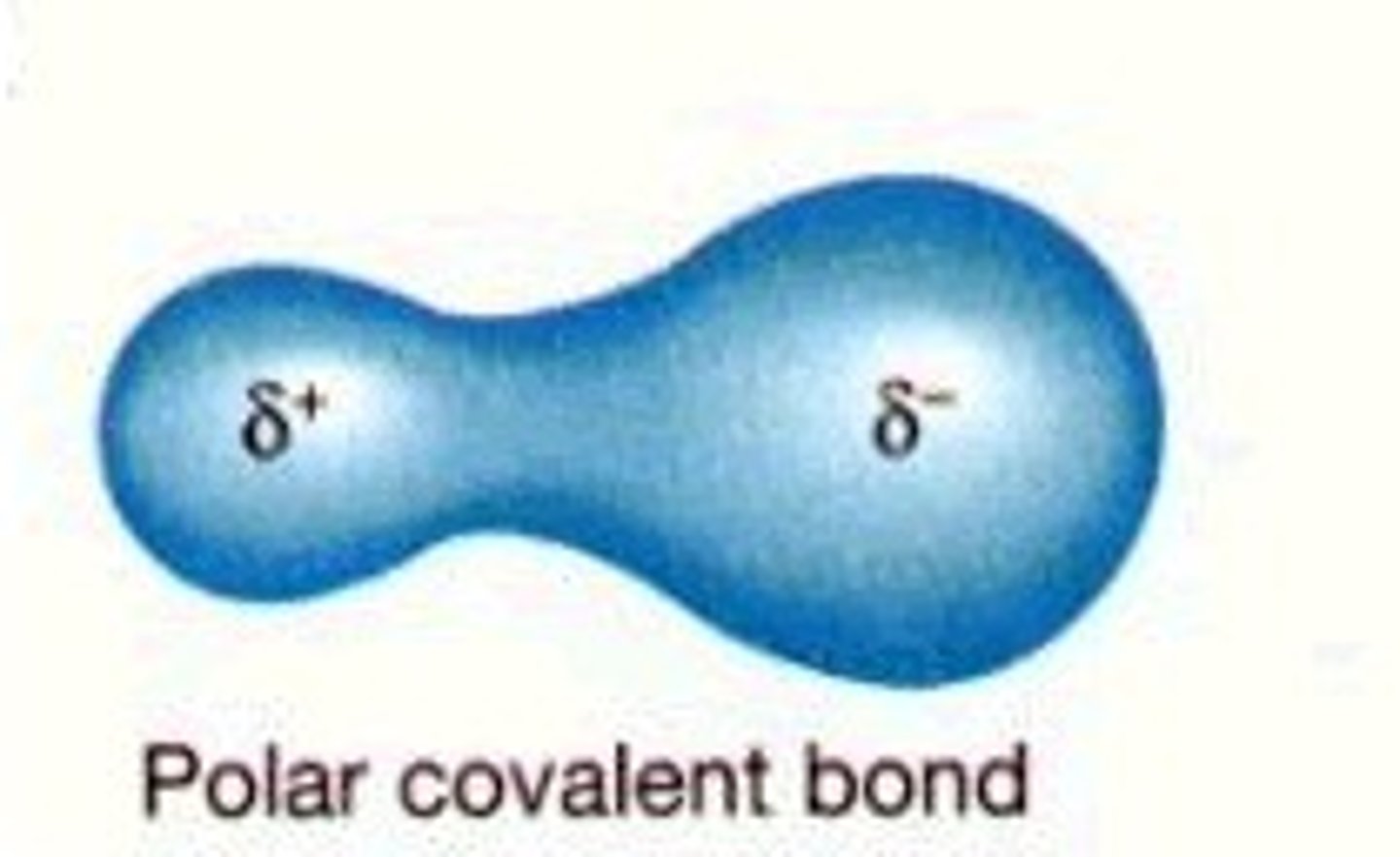
Polar Bond
In a polar bond, the electronegativity of the atoms will be different. An unequal sharing of electron pairs.
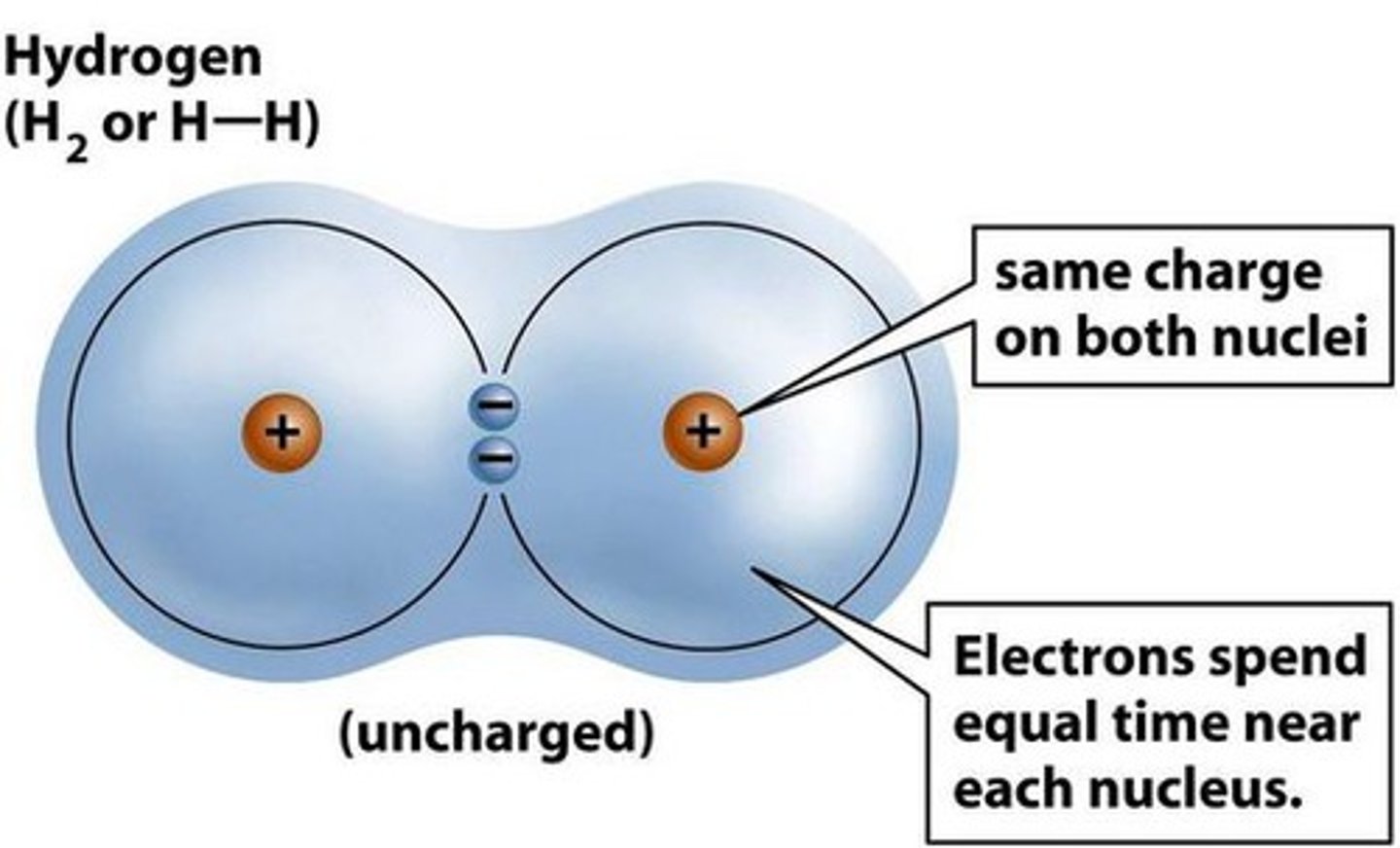
Non-polar Bond
For nonpolar bonds the electronegativity of the atoms will be equal.
Cohesion
attraction between like molecules. Water is cohesive, meaning, it stays together.
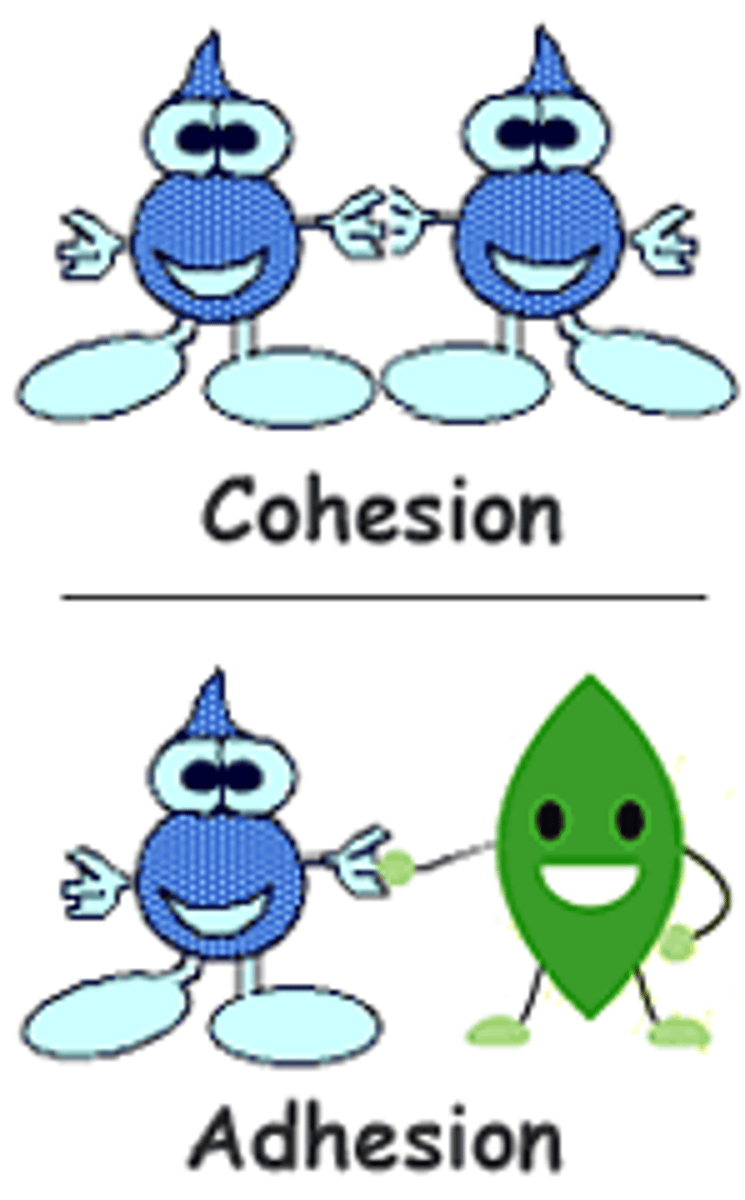
Adhesion
attraction between unlike molecules. In most cases between a liquid and a solid. ex. a meniscus where the waters partial positive charge adheres to the glass' negative charge.
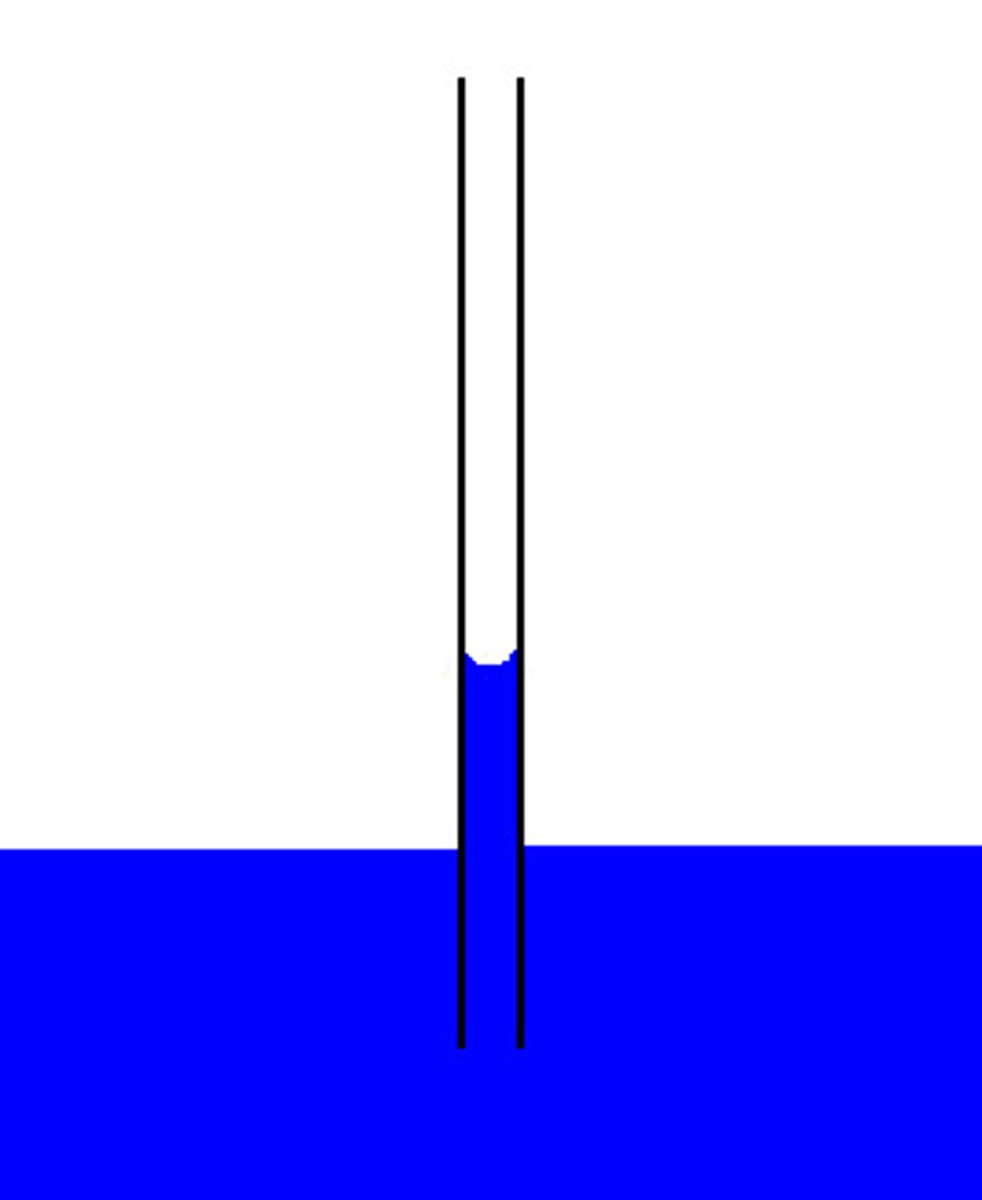
Surface Tension
a cohesive force caused by attraction between the molecules at the surface of a liquid.

Specific Heat
the amount of energy required to raise the temeprature of 1 gram of a substance by 1 degree celsius. Water has a high specific heat.
Describe the four emergent properties of water that allow Earth to sustain life.
1. High Specific Heat/Moderation of Temp.
2. Solvent
3. Expansion when freezing/Ice floats
4. Cohesion and Adhesion
Moderation of Temperature
Water is able to absorb or release large amounts of heat with only a slight change in its own temperature
Expansion Upon Freezing
When water freezes, it expands. Therefore ice floats. If ice didn't float it would kill everything at the bottom of the ocean and lakes.
Predict the effects of breaking the different bonds associated with water
The water-hydrogen bond is a weak bond. It is strong enough, however, to be maintained during thermal fluctuations at, and below, ambient temperatures.
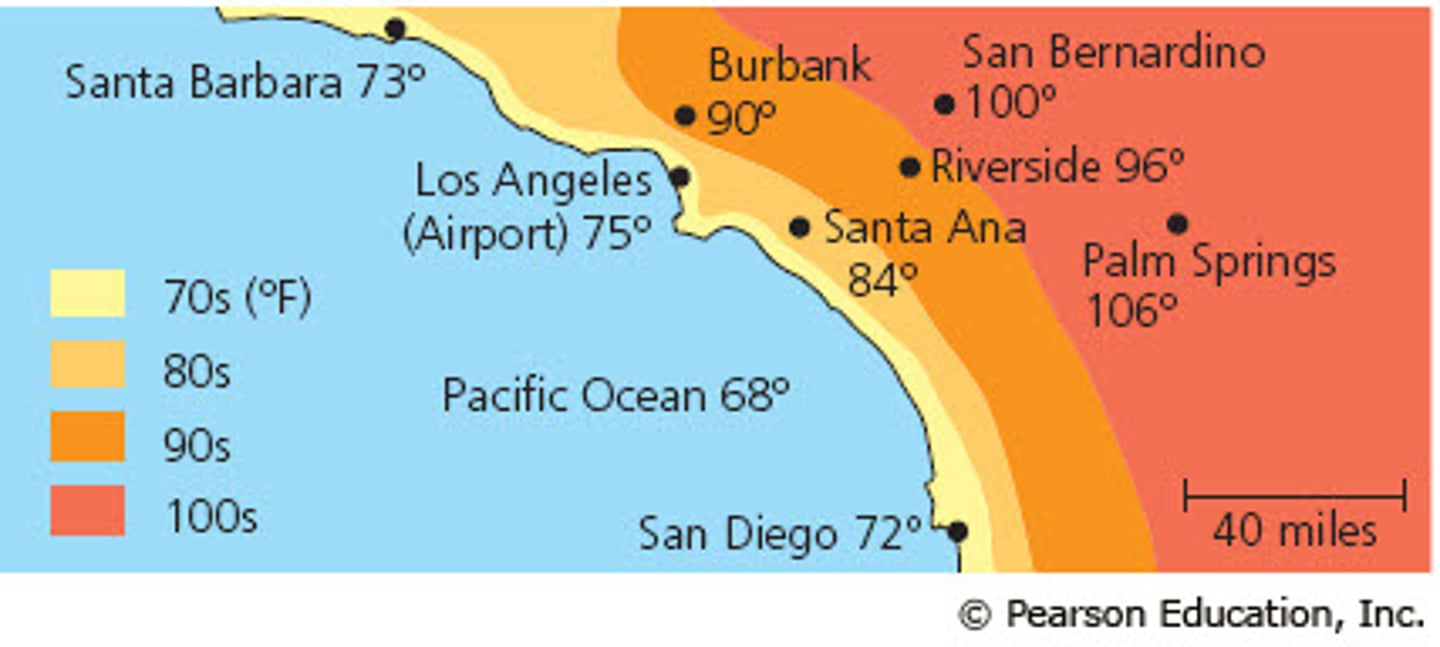
Explain how water's high specific heat helps to maintain temperatures
If a place such as california would be 75 at the shore and 90 away from the shore because the water uses the heat energy to heat itself up while colling the temperature around it.
Explain why carbon is considered the backbone of life
almost all molecules in a living organism (except water) contains carbon organic compounds. It also has 4 valence electrons which allows it to make up to 4 different bonds.
Explain the biological implication of isomers
Isomers are compounds with the same molecular formula but a different arranagement of the atoms. Because it has a different structure, it has a different function.
Identify key functional groups which play a major role in biological processes
Functional groups participate in chemical groups in a predictable monomer. They are H, N, O, P, and S.
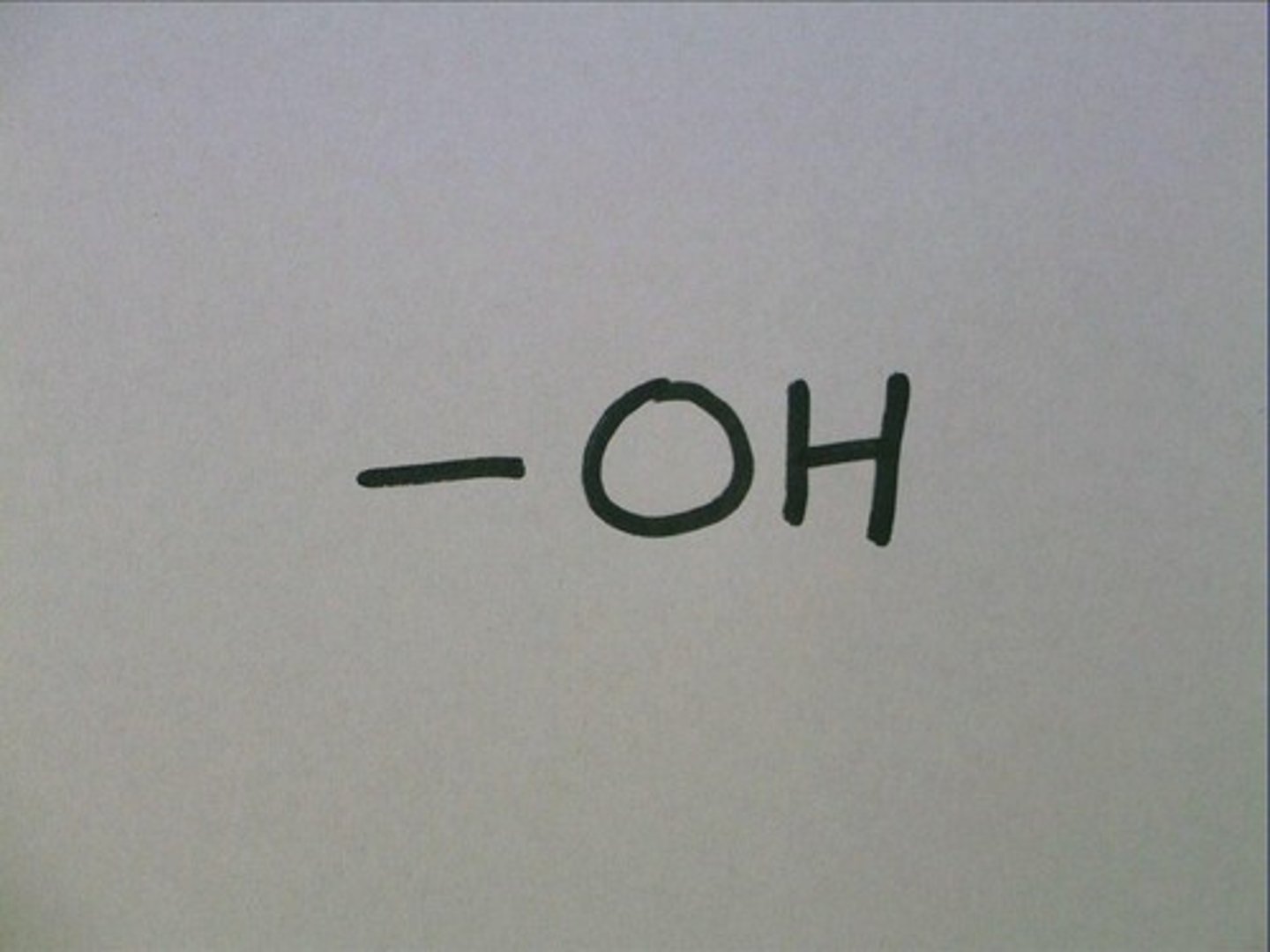
Hydroxyl
When a hydrogen is bonded to an oxygen. OH.
Carbonyl
Just a carbon double bonded with an oxygen. CO
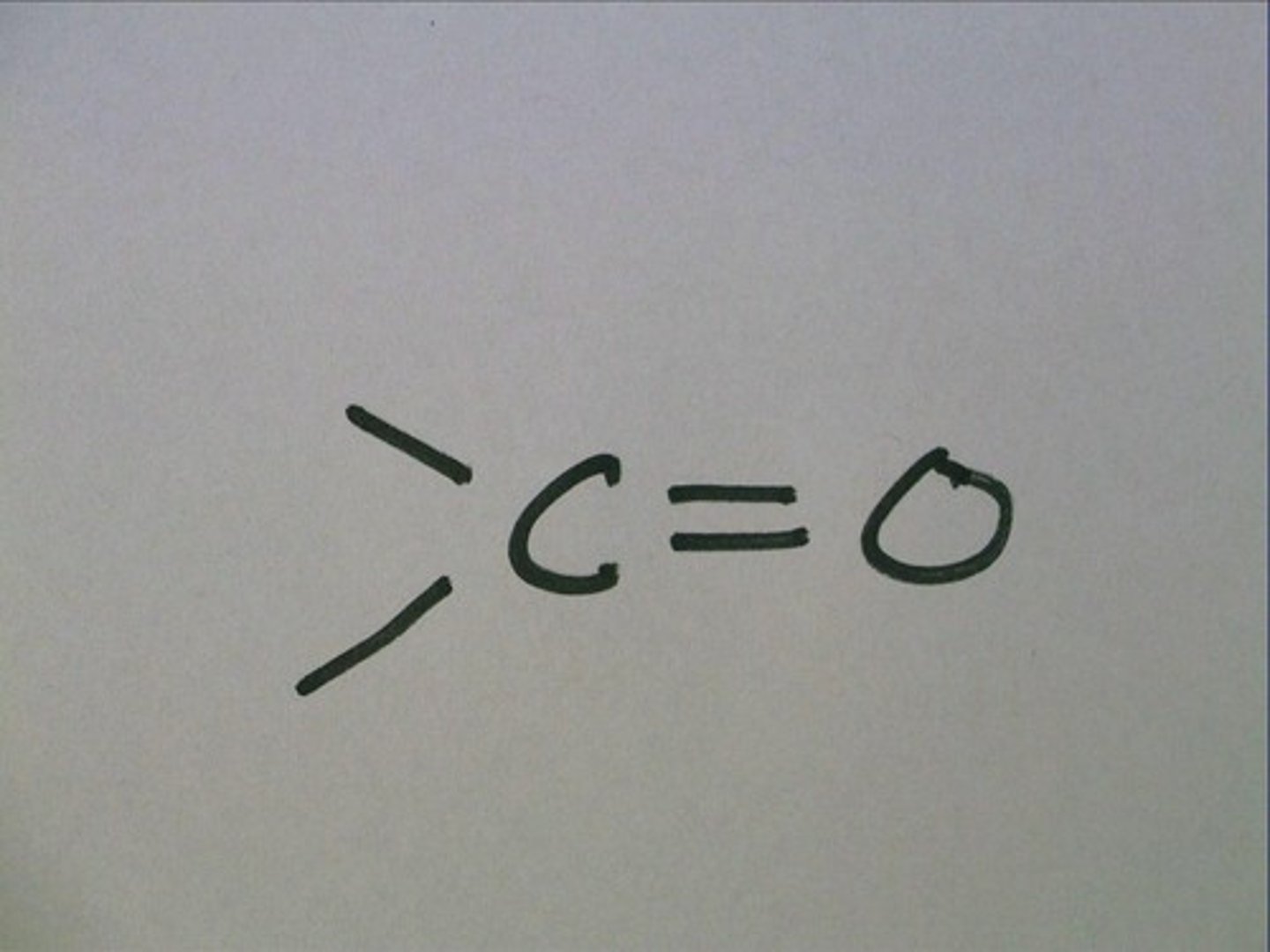
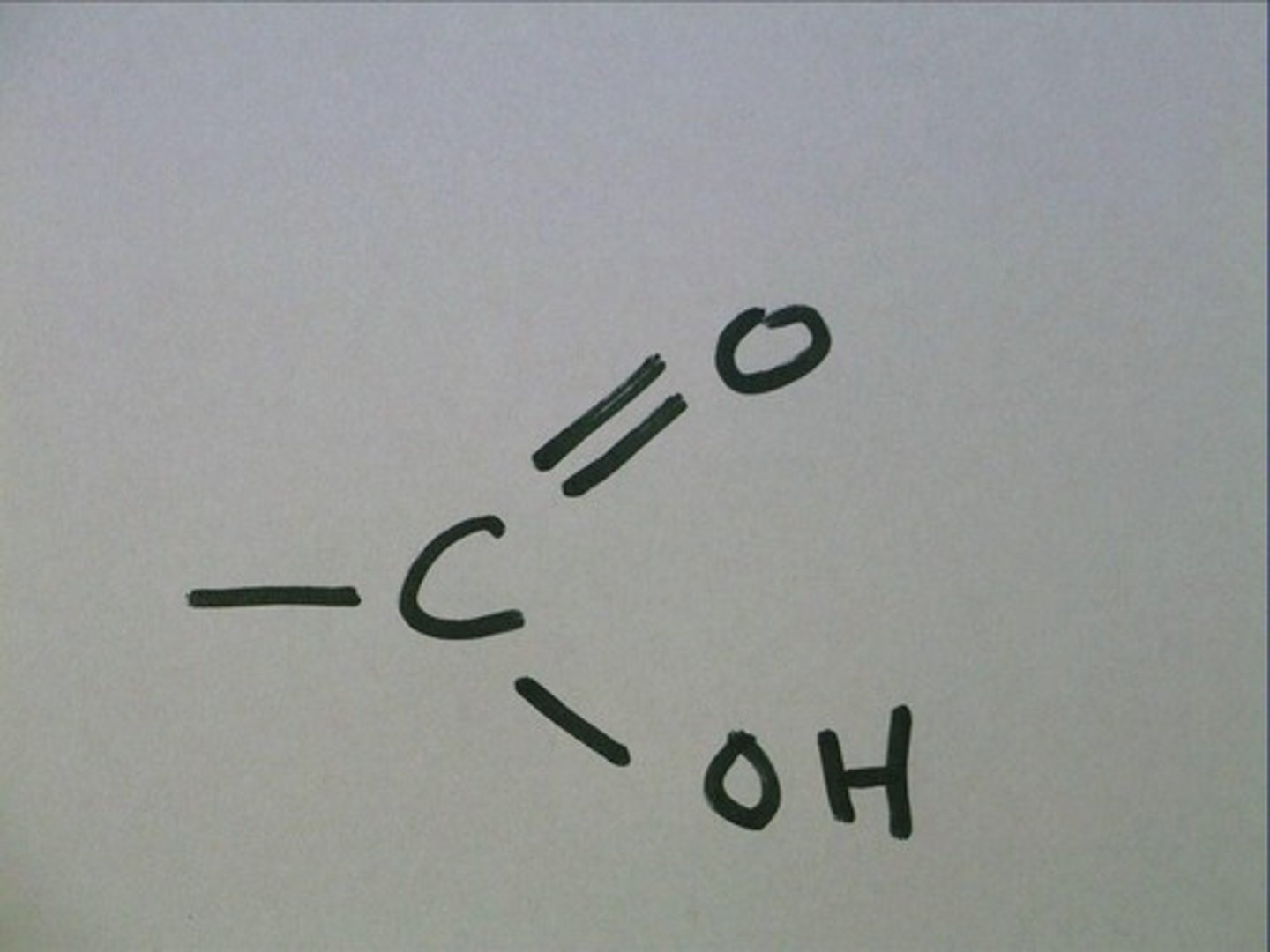
Carboxyl
A carboxyl group is when it is double bonded to an oxygen and also bonded with an OH. COOH.
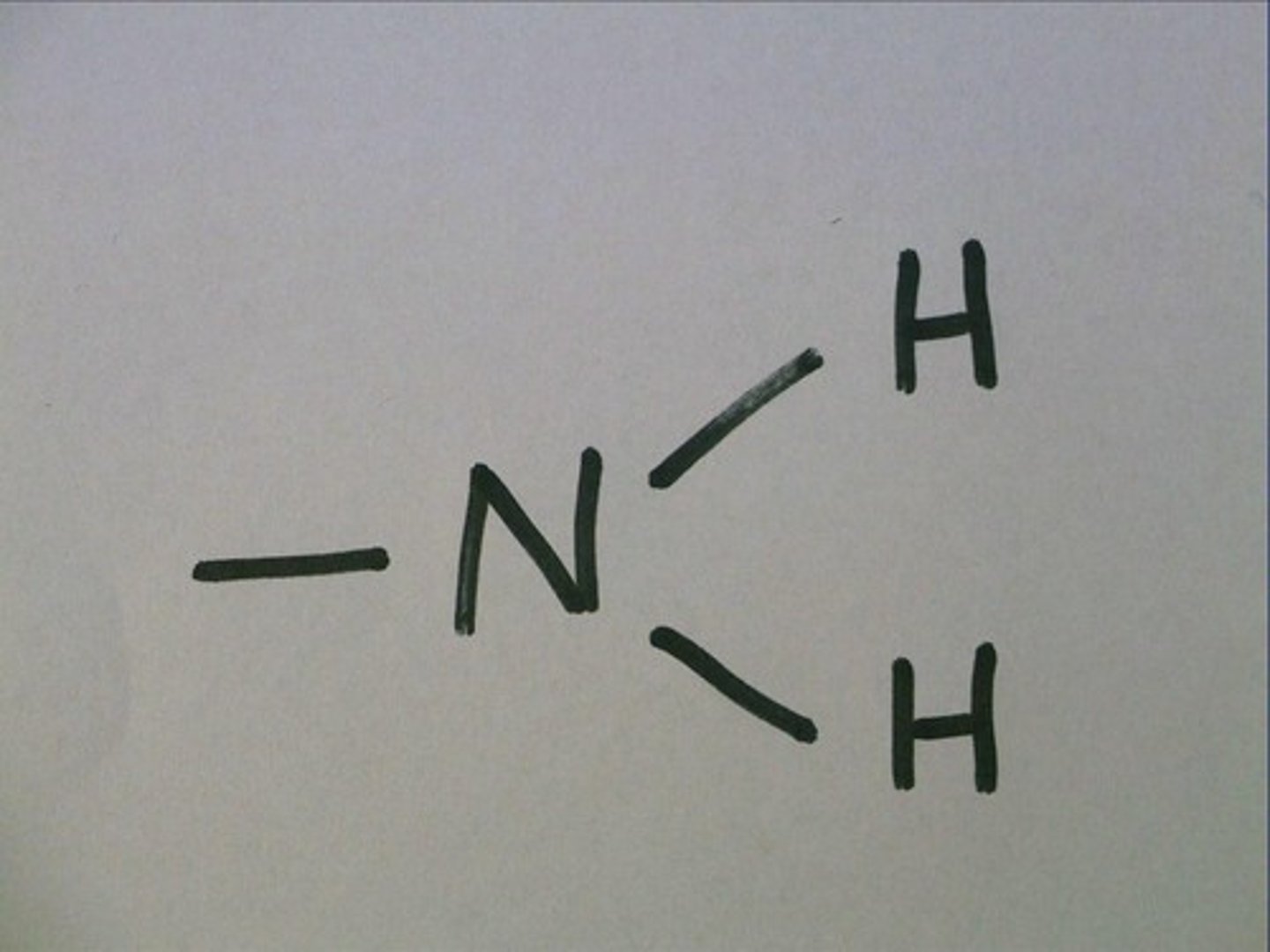
Amino
AN amino group is NH2 or one nitrogen bonded with two hydrogen.
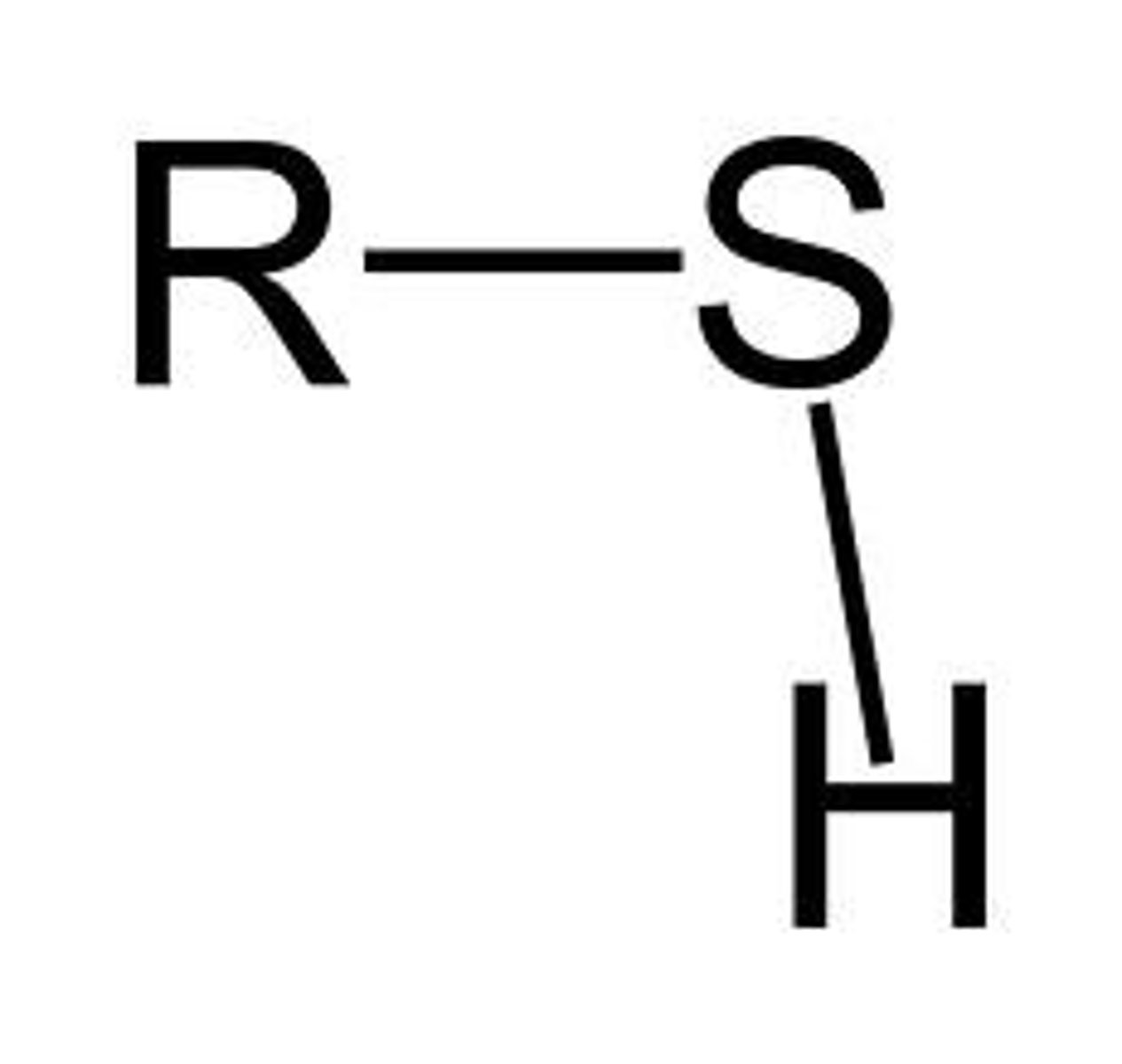
Sulfhydryl
The rest (R) of the chain is bonded with a sullfure thats bonded to a hydrogen. SH.
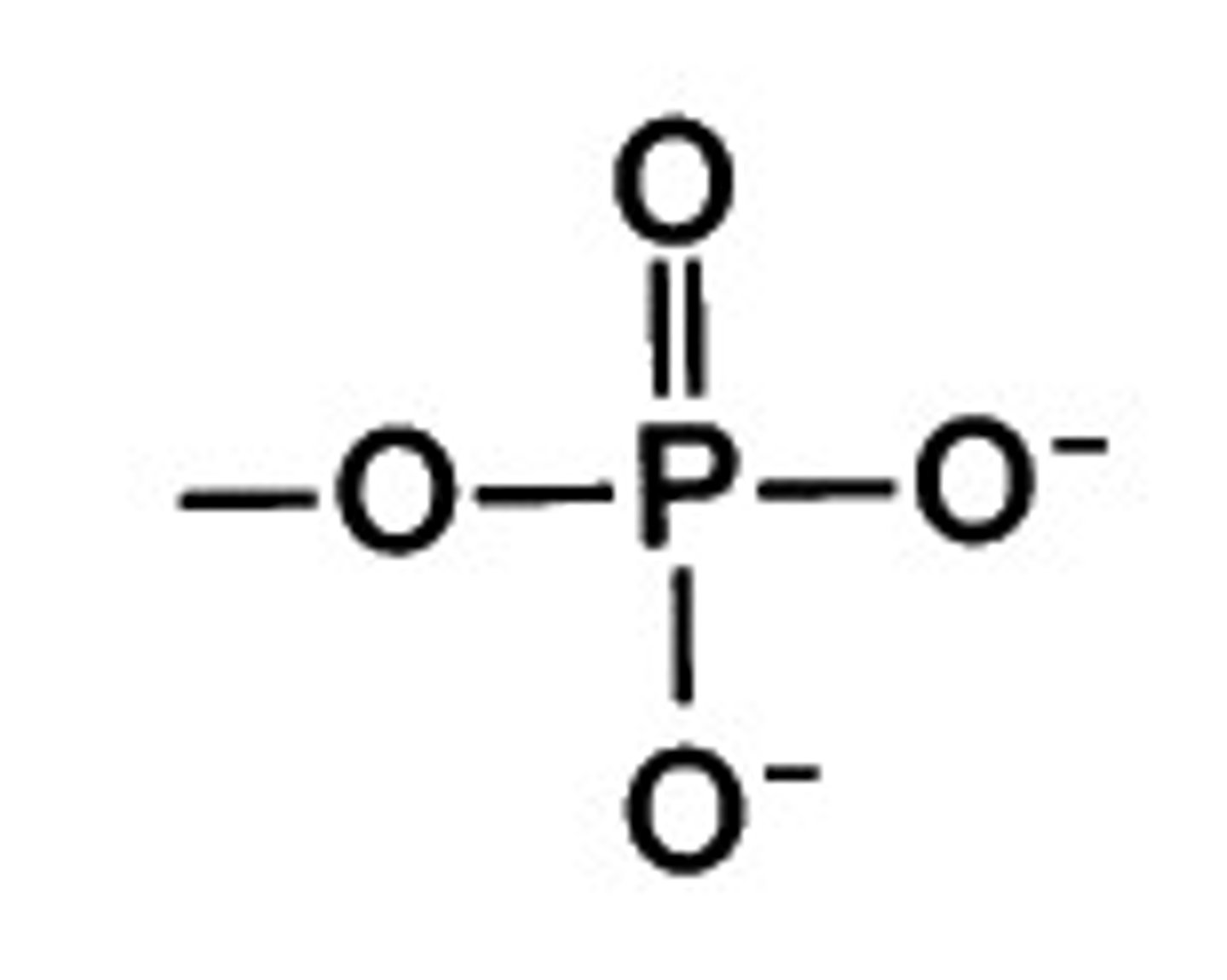
Phosphate
Phosphate group has four oxygen atoms bonded with one phosphate. PO4
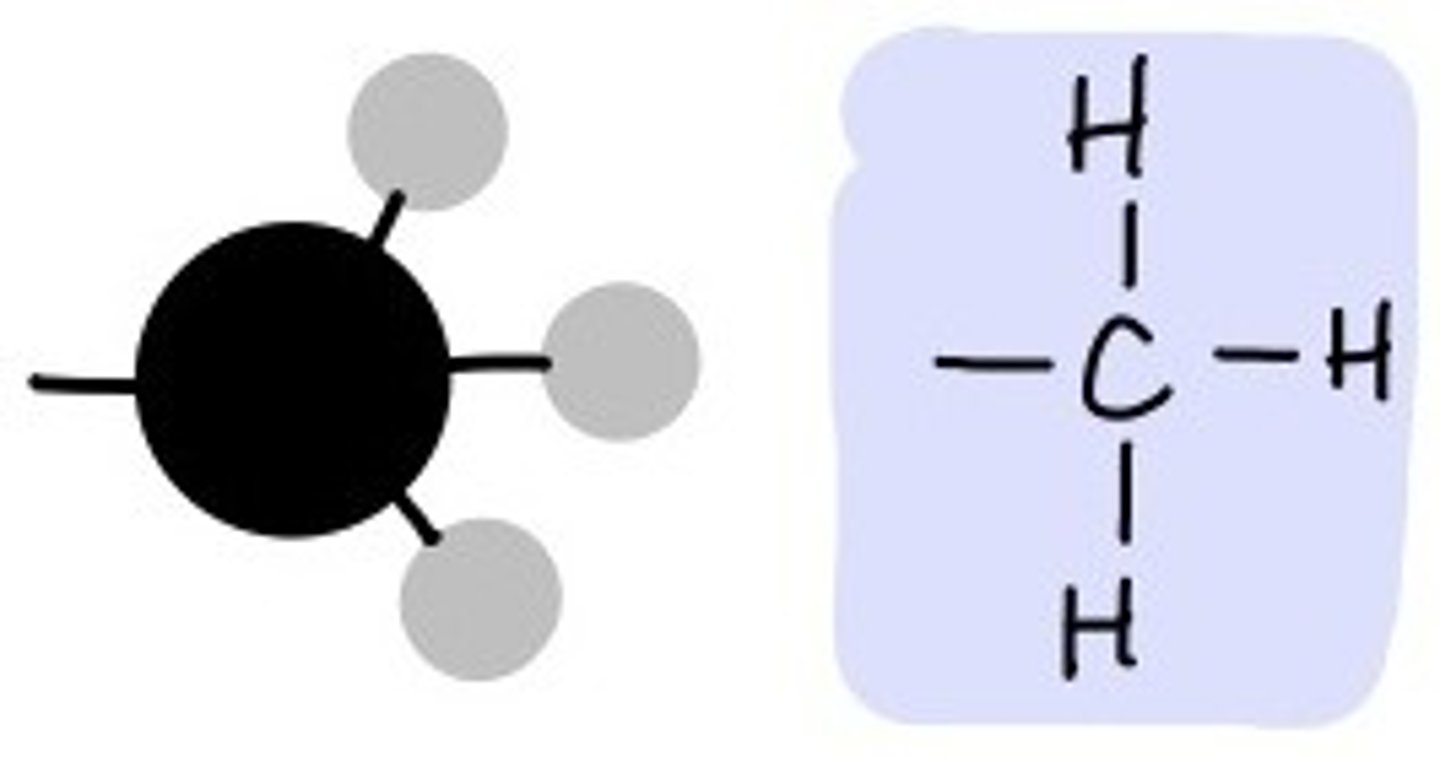
Methyl
It is a carbon atom bonded to three hydrogen atoms. CH3.
Proteins
They are responsible for almost everything an organims does. They are structurally diverse which means they have a wide range of functions. ex. enzymes, storage, transport, defense (antibodies), hormones, receptors, strcuture.
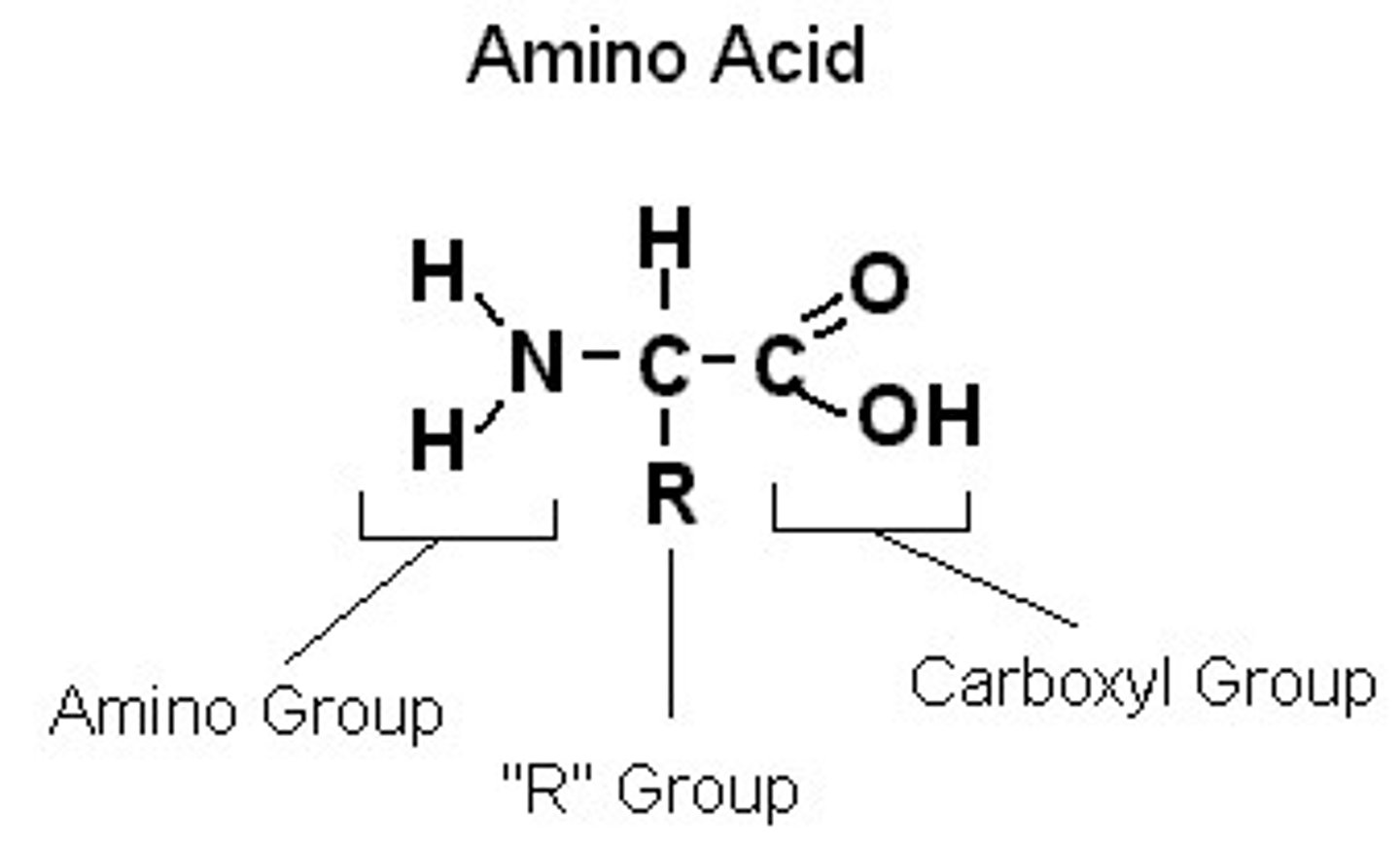
Amino Acids
Building blocks of proteins. They all share the same core structure, which is an amino group, a carboxyl group, and an R group (this is what makes it unique and makes it have its own unique function). They are the monomer of proteins.
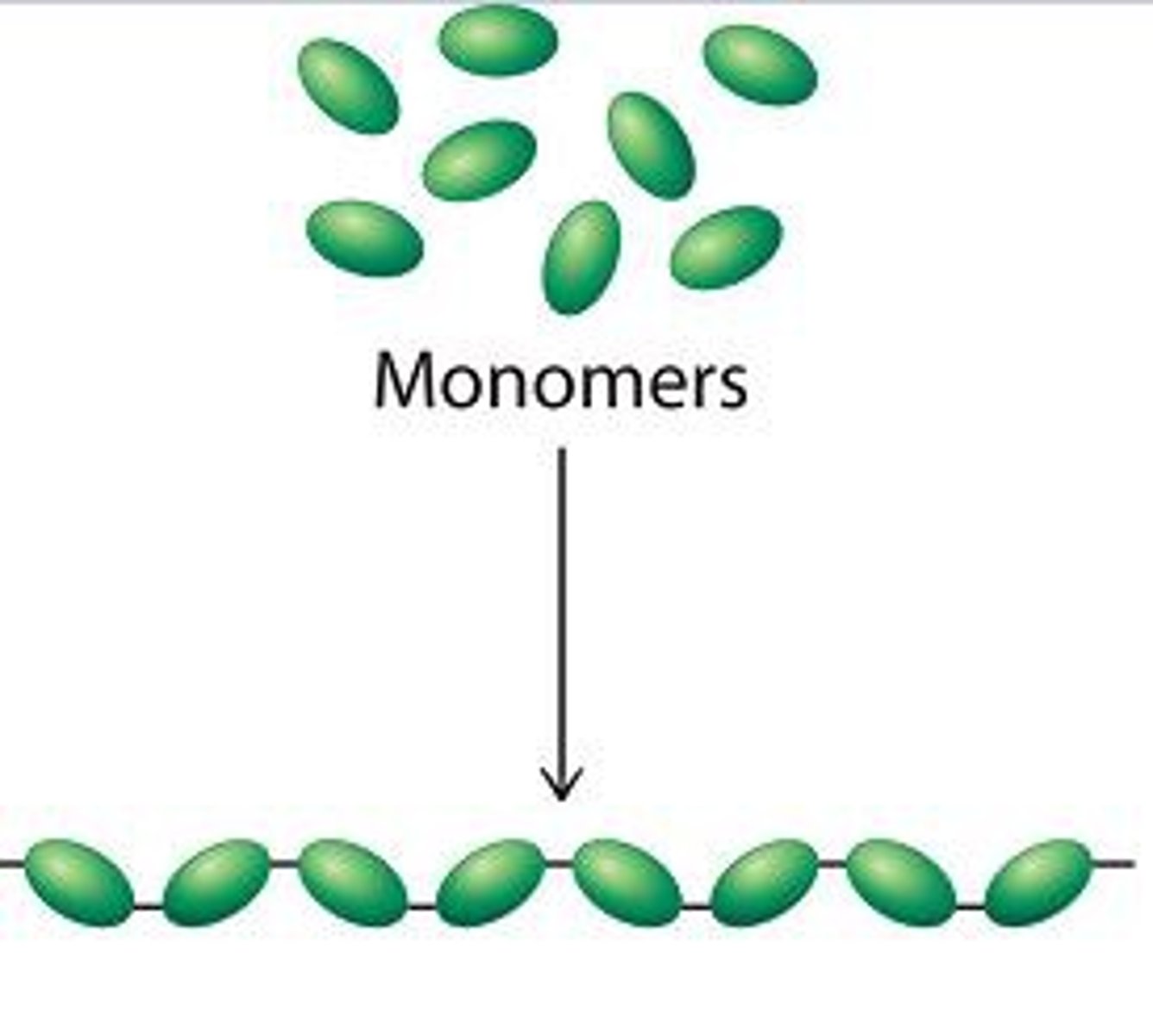
Polymerization
the bonding together of monomers. Amino acids (protein monomer) come together through polymerization to form proteins.
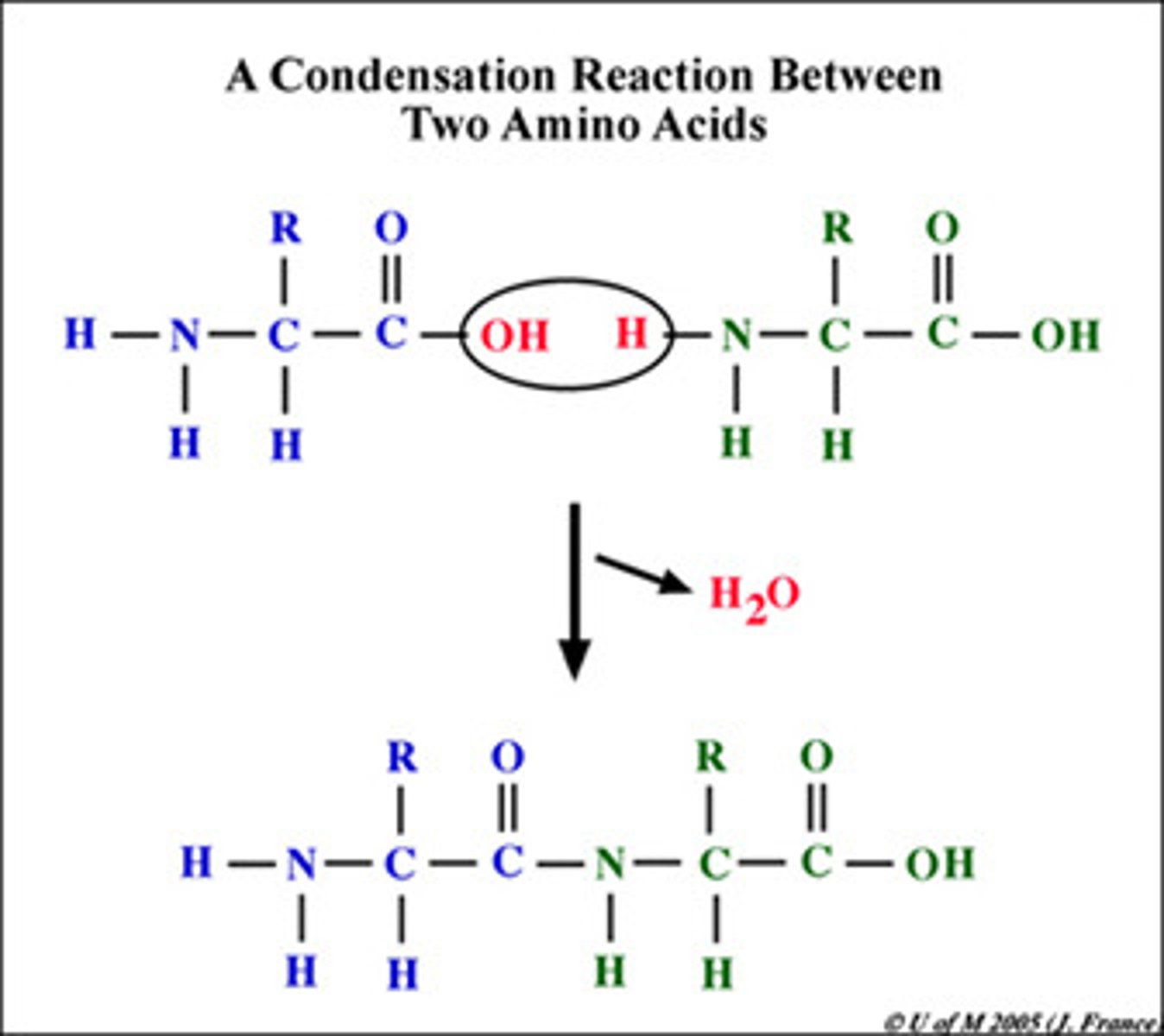
Condensation Reaction
Also known as a dehydration reaction. These bonds form and result in the loss of a water molecule.
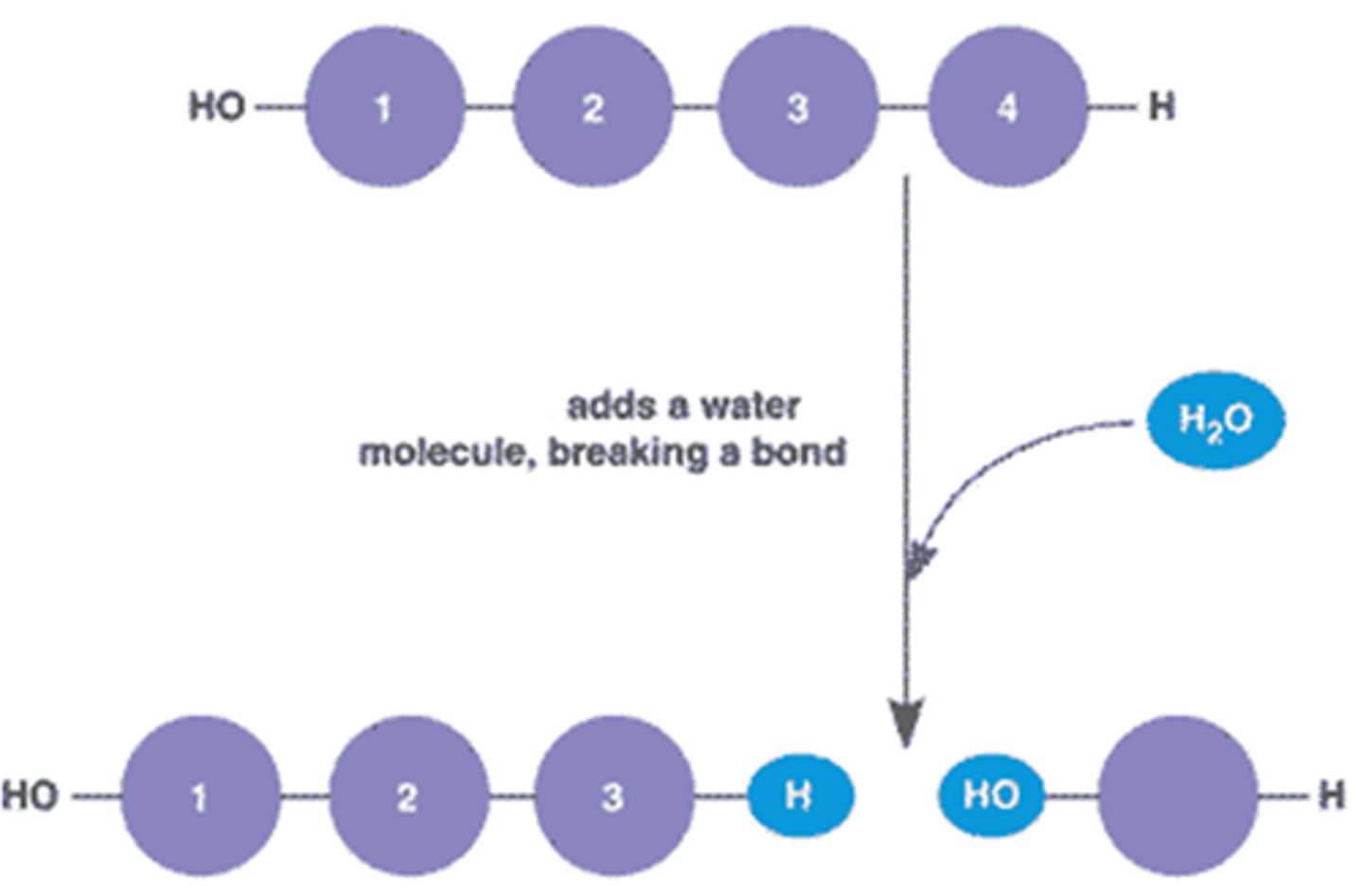
Hydrolysis
The opposite of a condensation reaction. It breaks polymers apart by adding a water molecule.
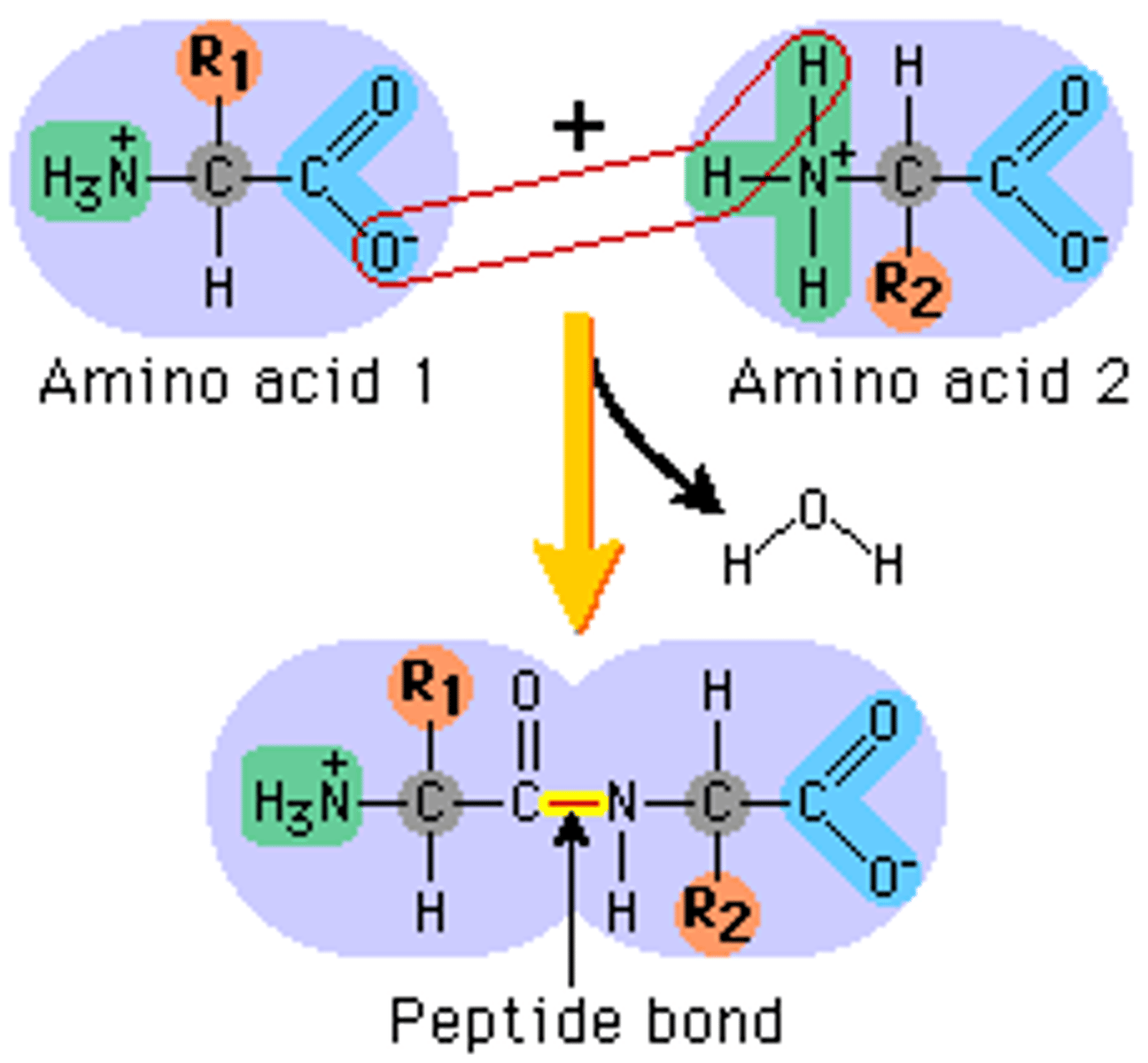
Peptide Bond
When the carboxyl group of one amino acid reacts with the amino acid group of another amino acid, a strong covalent bond called a peptide bond forms.
Oligopeptide
(few peptides) When fewer than 50 amino acids are linked together.
Polypeptide
(many peptides) 50 or more amino acids are linked together.
The four protein structures are?
1. Primary
2. Secondary
3. Tertiary
4. Quaternary
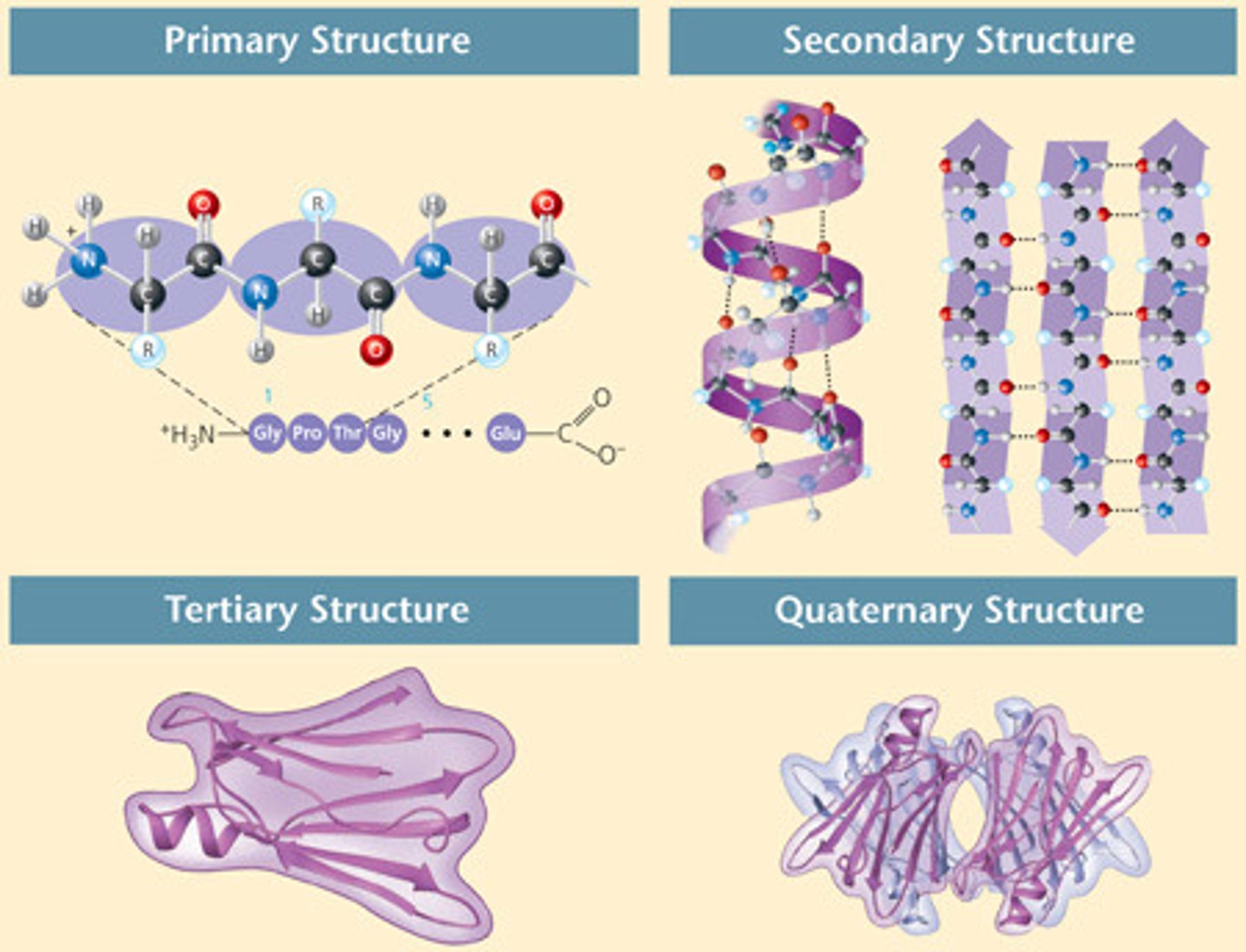
Primary Structure
The unique sequence of amino acids making up the protein is considered its primary structure. ex. a normal sequence would give normal red blood cells. But a single change in sequence could leave you having sickled red blood cells. A proteins primary structure is fundamental to its function.
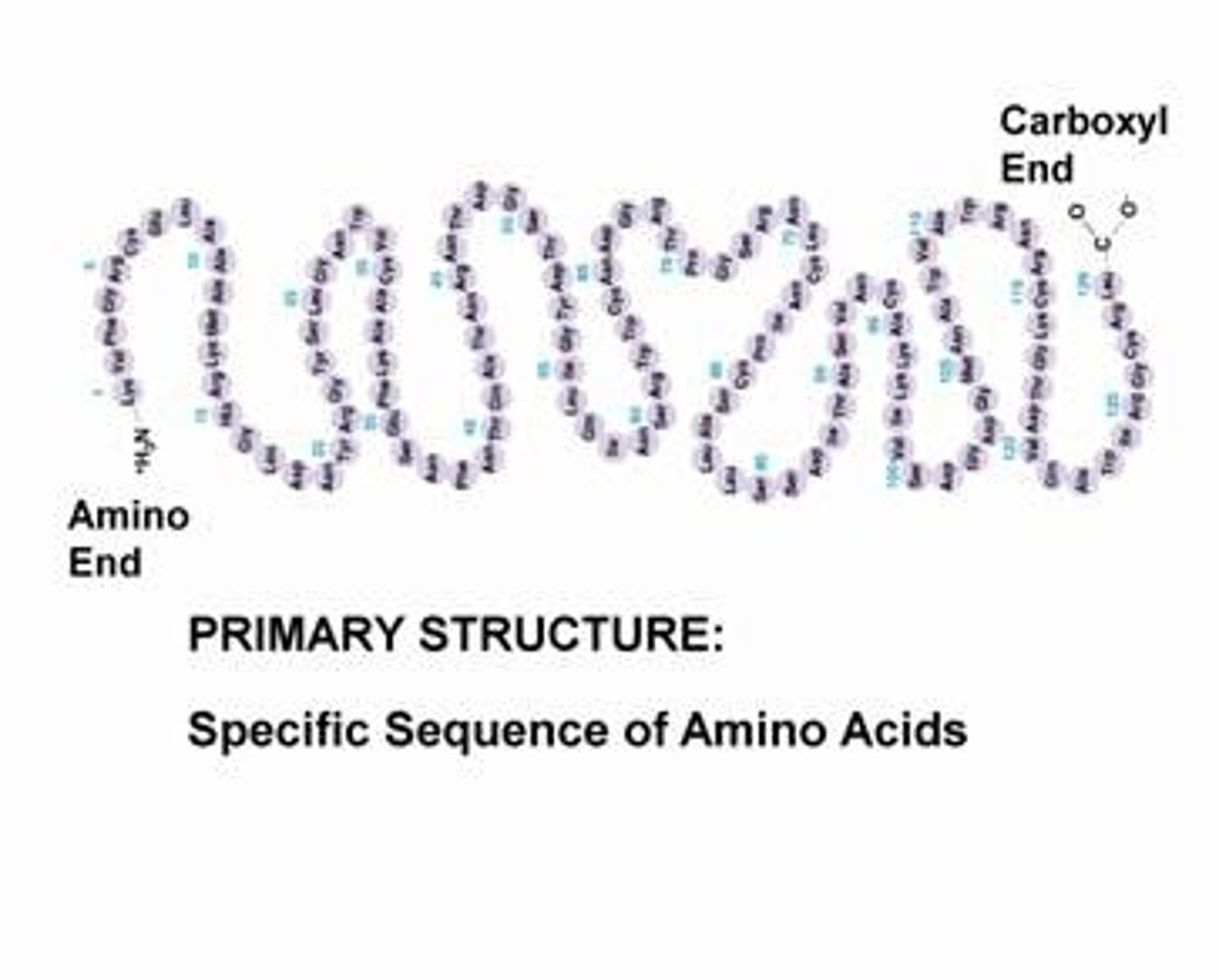
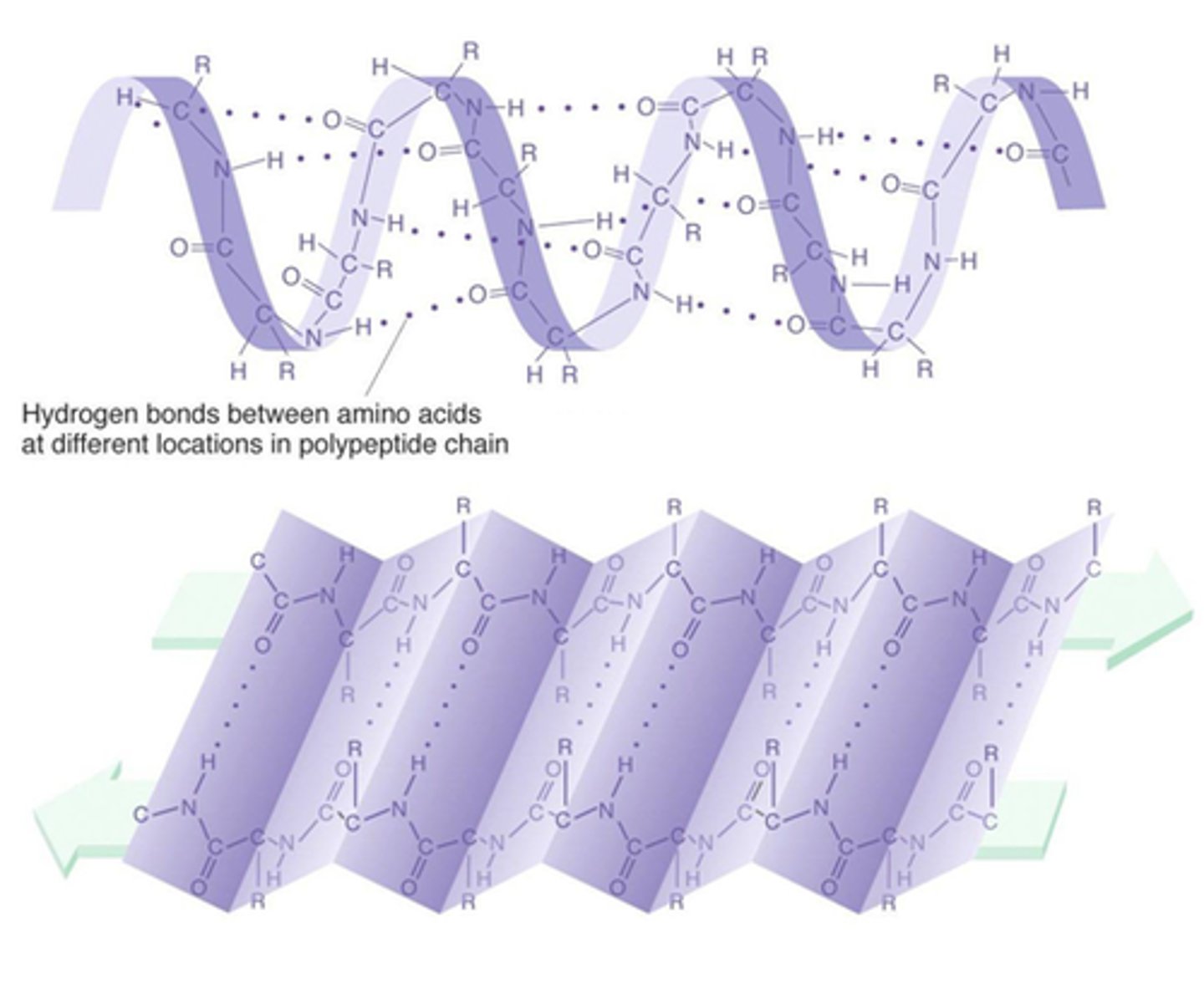
Secondary Structure
Is created in part by interactions between functional groups in the peptide-bonded backbone. The hydrogen bonding of close amino and carbonyl groups either makes it a helix or a pleated sheet. refers to the coiling or folding of a polypeptide chain that gives the protein its 3-D shape.
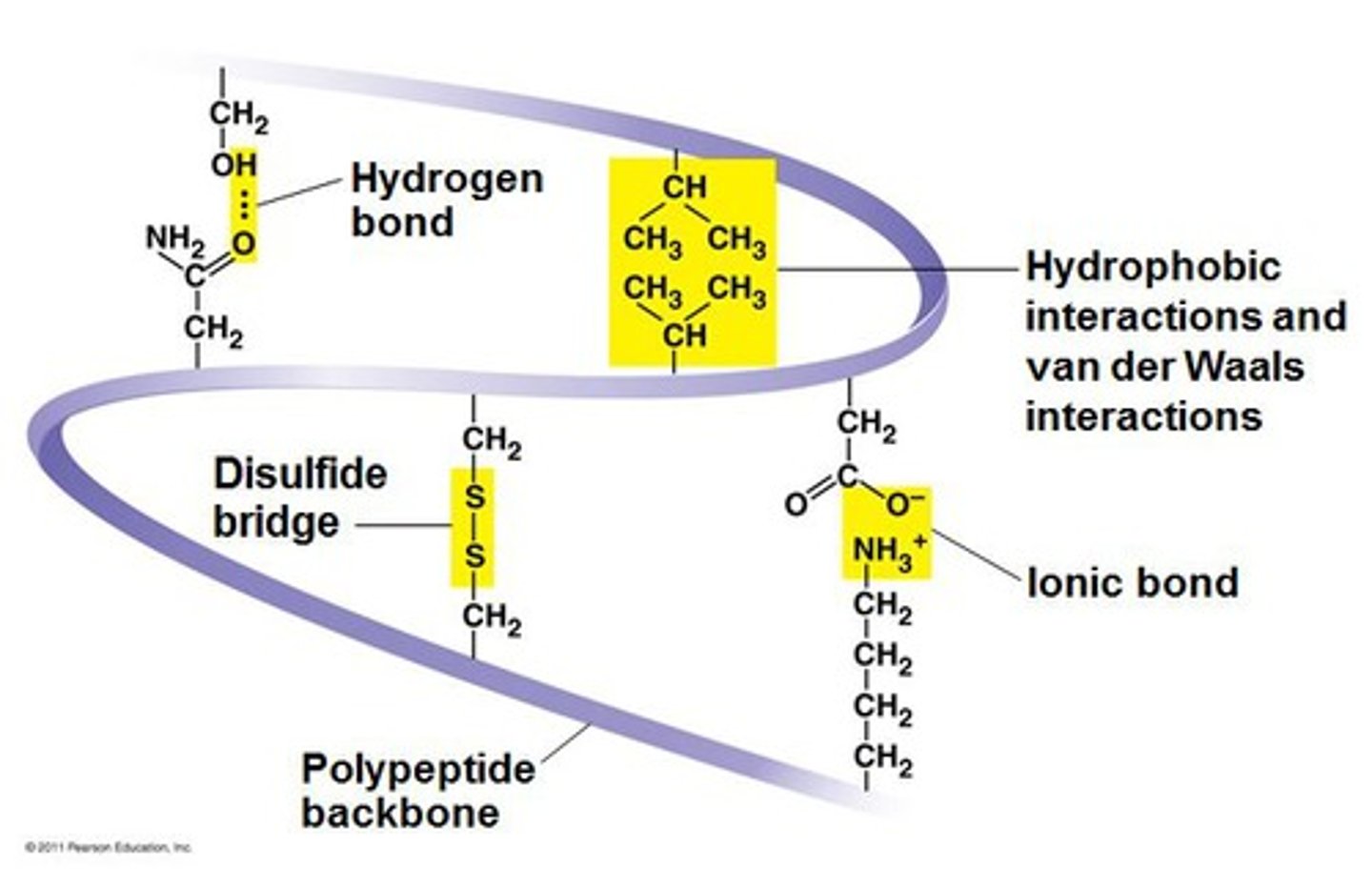
Tertiary Structure
Interactions between R-groups. There could be hydrogen bonding, hydrophobic interactions, covalent bonding, and ionic bonding between R groups. Determines its 3D shape.
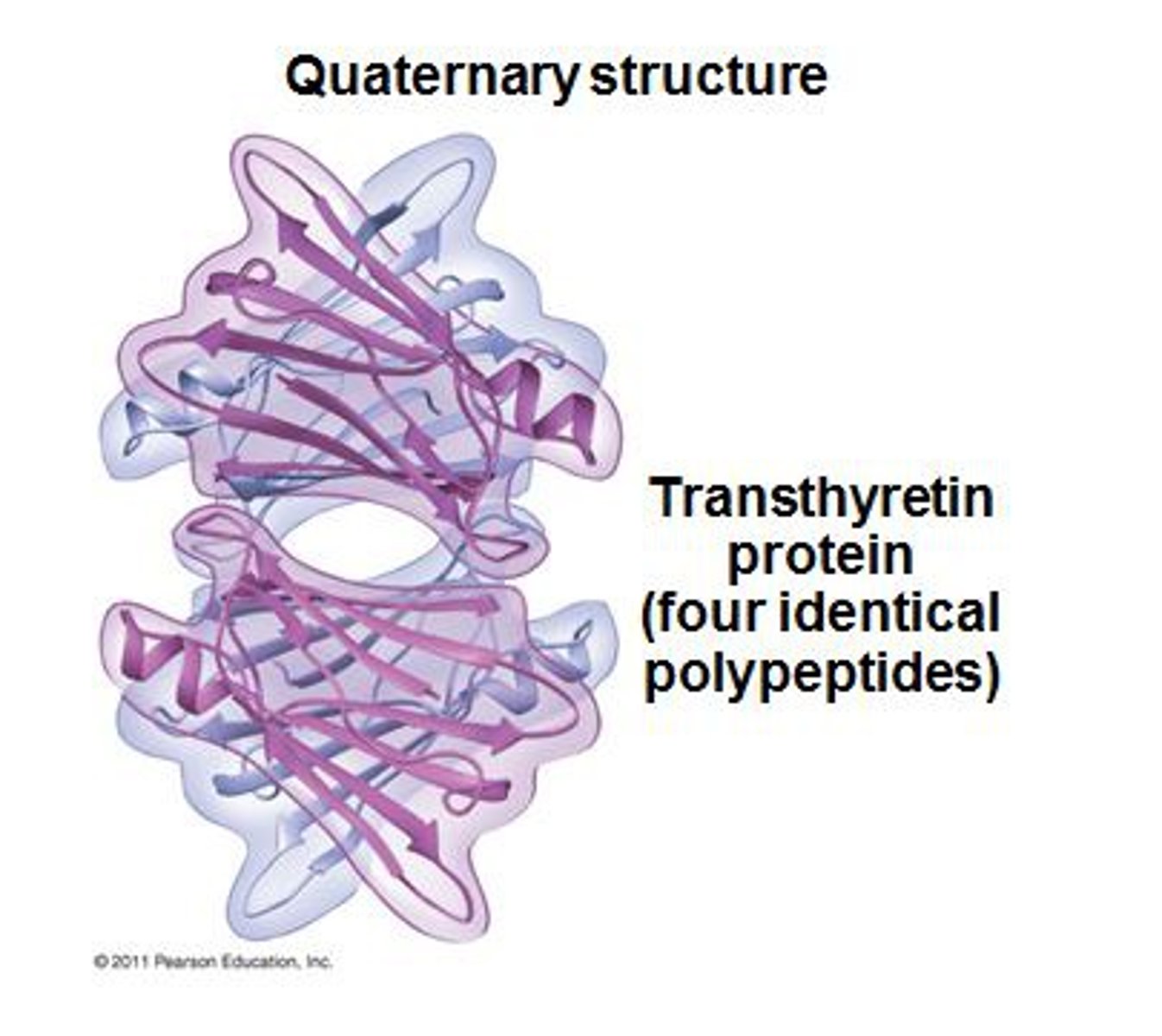
Quaternary Structure
refers to the structure of a protein macromolecule formed by interactions between multiple polypeptide chains. Basically multiple polypeptides coming together. Each polypeptide chain is referred to as a subunit. Proteins with quaternary structure may consist of more than one of the same type of protein subunit.
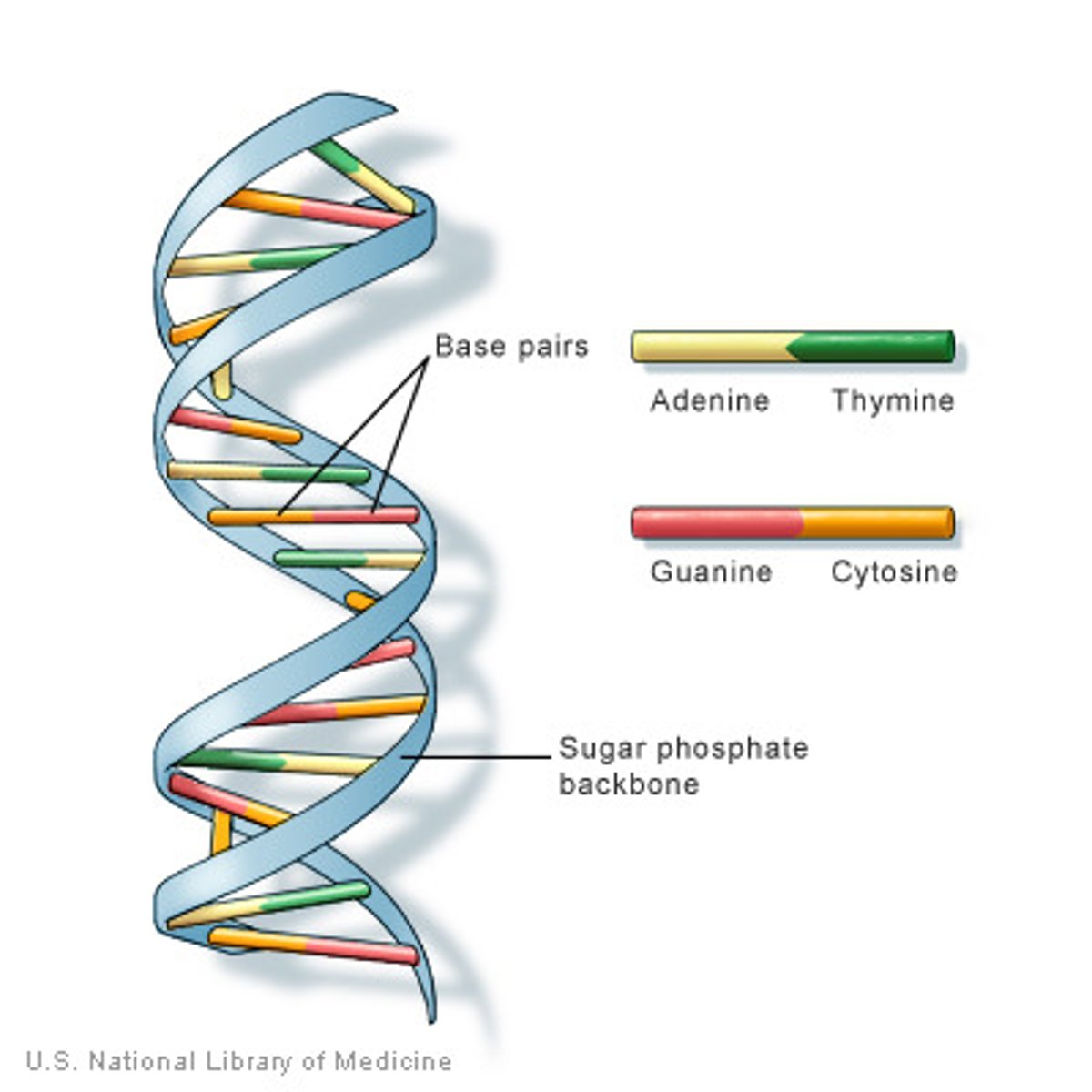
DNA
Deoxyribonucleic acid stores genetic information and creates instructions for life. Made up of deoxyribonucleotides (monomer). The sugar is deoxy meaning without oxygen, so it only has one H. (A,T, and G,C base pairs)
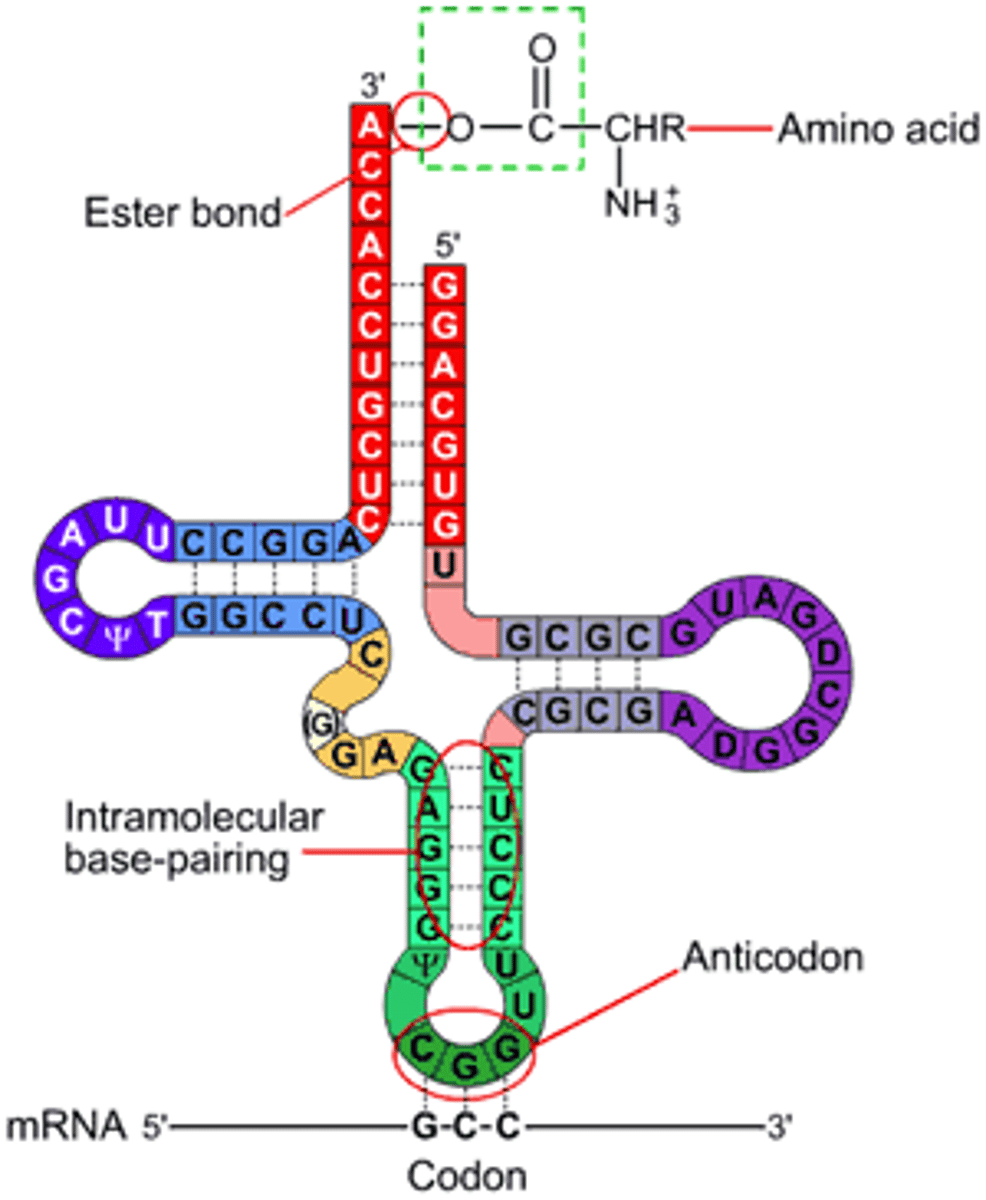
RNA
Ribonucleic Acid takes the instructions and translates them into proteins. Made up of ribonucleotides (monomer). The sugar is ribose meaning with two OH. (A,U, and G,C). It has a hydroxyl (OH) unlike DNA.
Nucleic Acid
is a polymer made up of nucleotides (monomers).
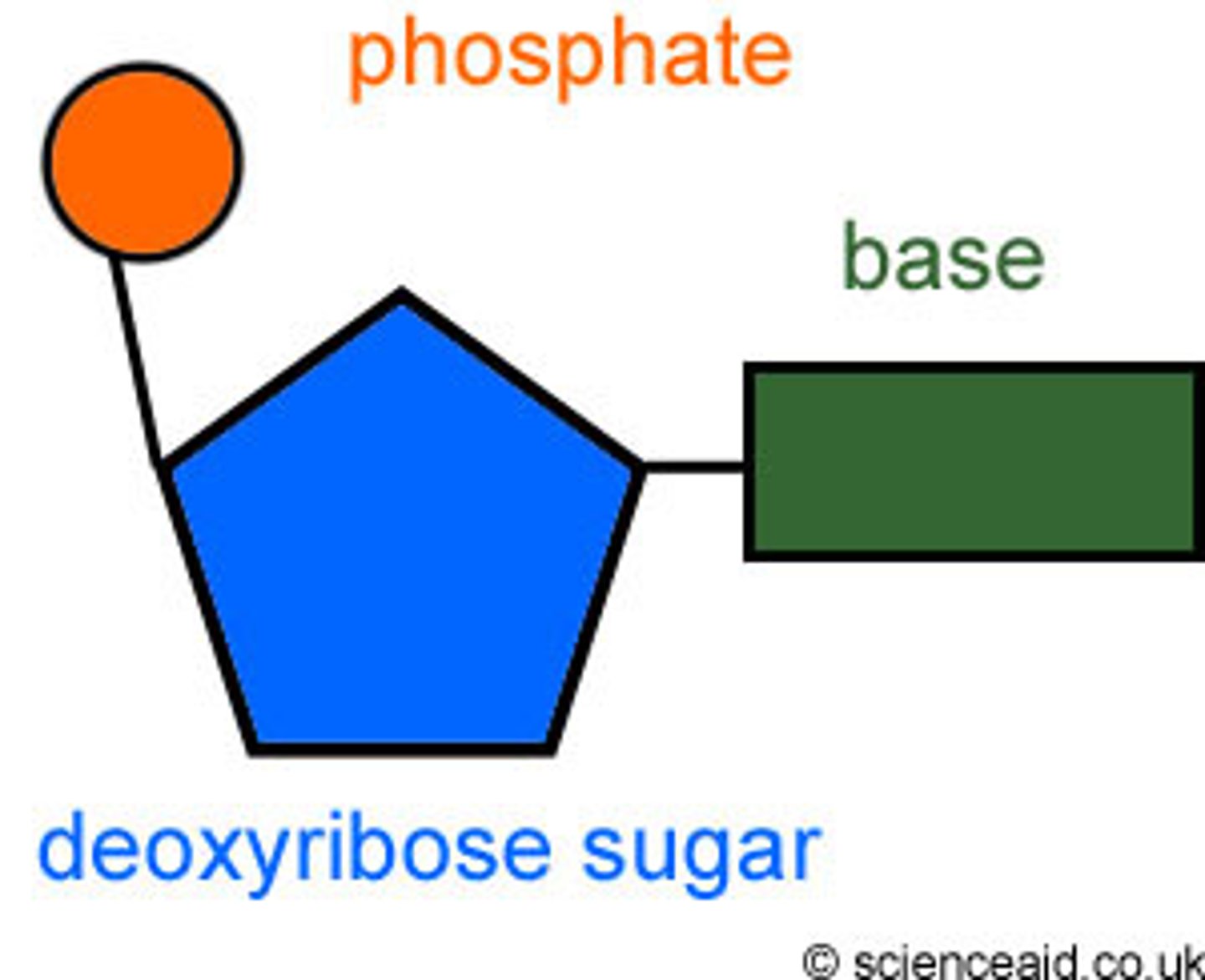
Nucleotide
composed of a phosphate group, a 5-carbon sugar, and a nitrogenous base.
Nucleotide Polymerization
Nucleotides bond together with other nucleotides through a condensation reaction, meaning a water is released. This reaction forms a covalent bond called a phosphodiester linkage. Phosphodiester linkages that join ribonucleotides together form RNA. Phosphodiester linkages that put deoxyribonucleotides together form DNA.
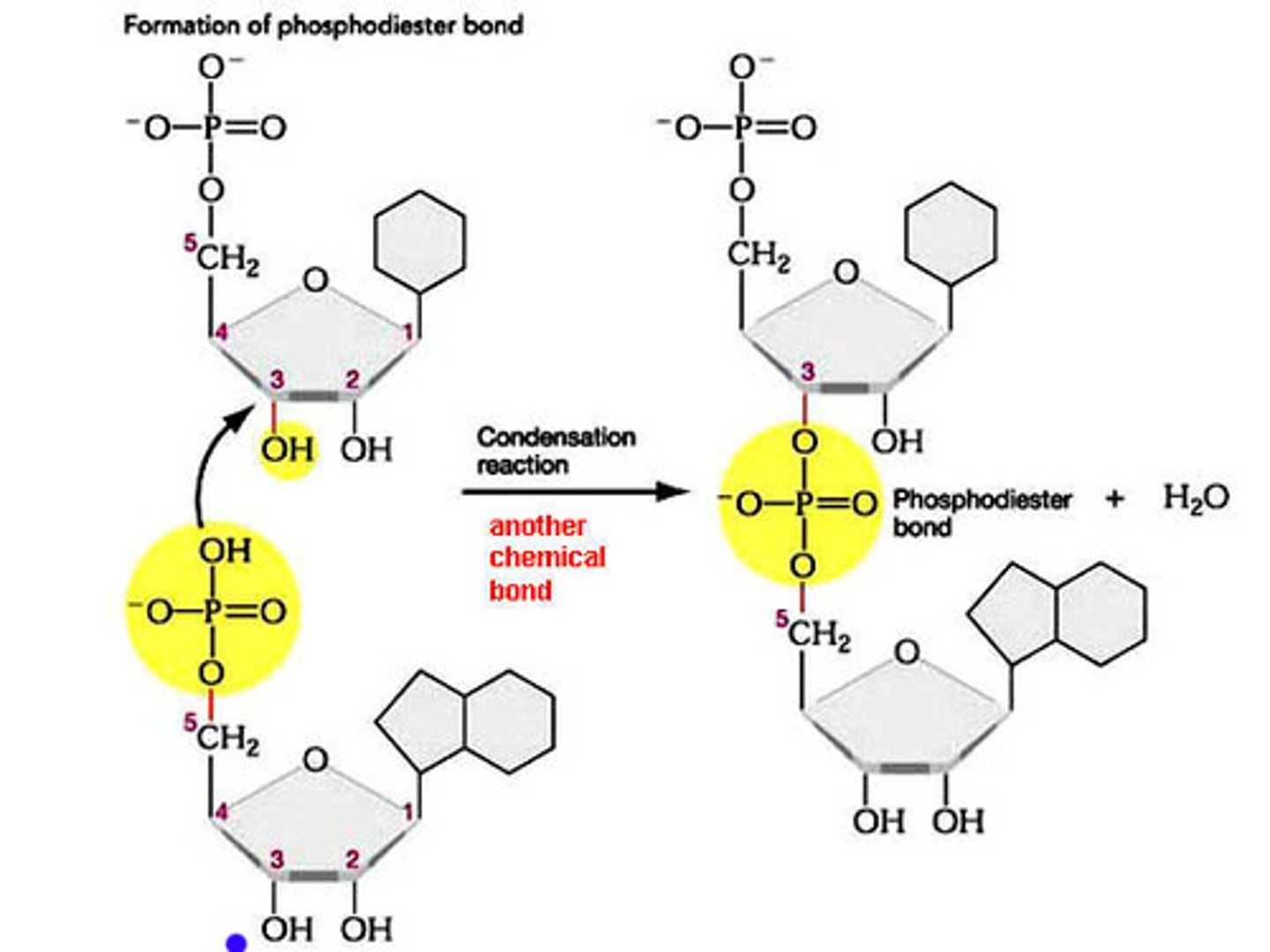
Nucleotide Structure
Nucleotides polymerize to form nucleic acids through formation of phosphodiester linkages between the 3" hydroxyl on one nucleotide and the 5" phosphate on the other.
Polymerization requires energy
ATP which is energy that comes from an activated nucleotide.
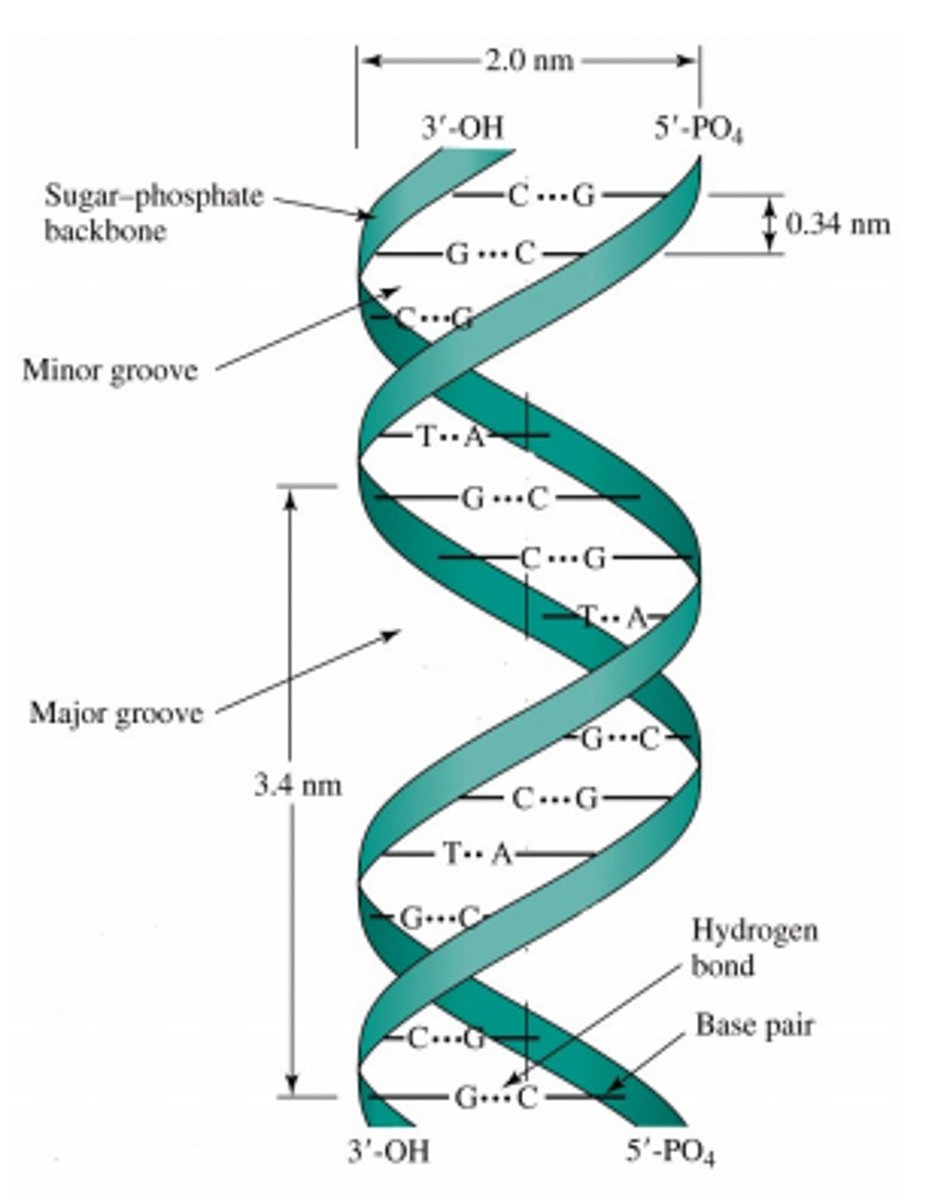
G and C
have three hydrogen bonds
A and T
have two hydrogen bonds
In double stranded DNA,
backbones must run anti-parallel. Meaning 5-3 and 3-5.
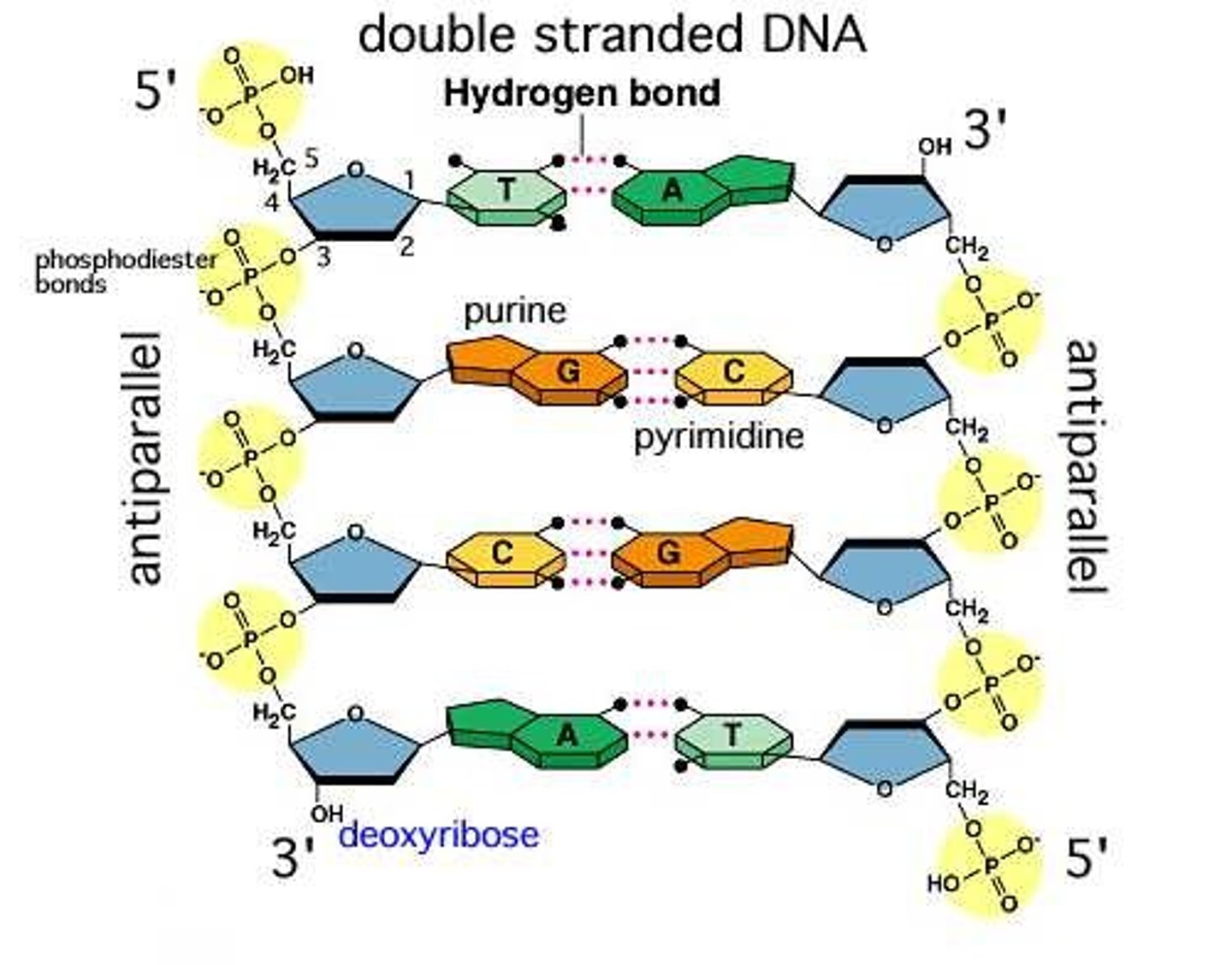
DNA's secondary structure
consists of two antiparallel strands twisted into a double helix. This was found out by Watsin and Crick.
RNA Structure
Contains Uracil instead of Thymine. Usually single-stranded, but can fold in its form of tertiary structure.
The monomers of carbohydrates are
monosaccharide is the primary monomer(one sugar), oligosaccharide ("few sugars"), and the large polymers called polysaccarides ("many sugars").
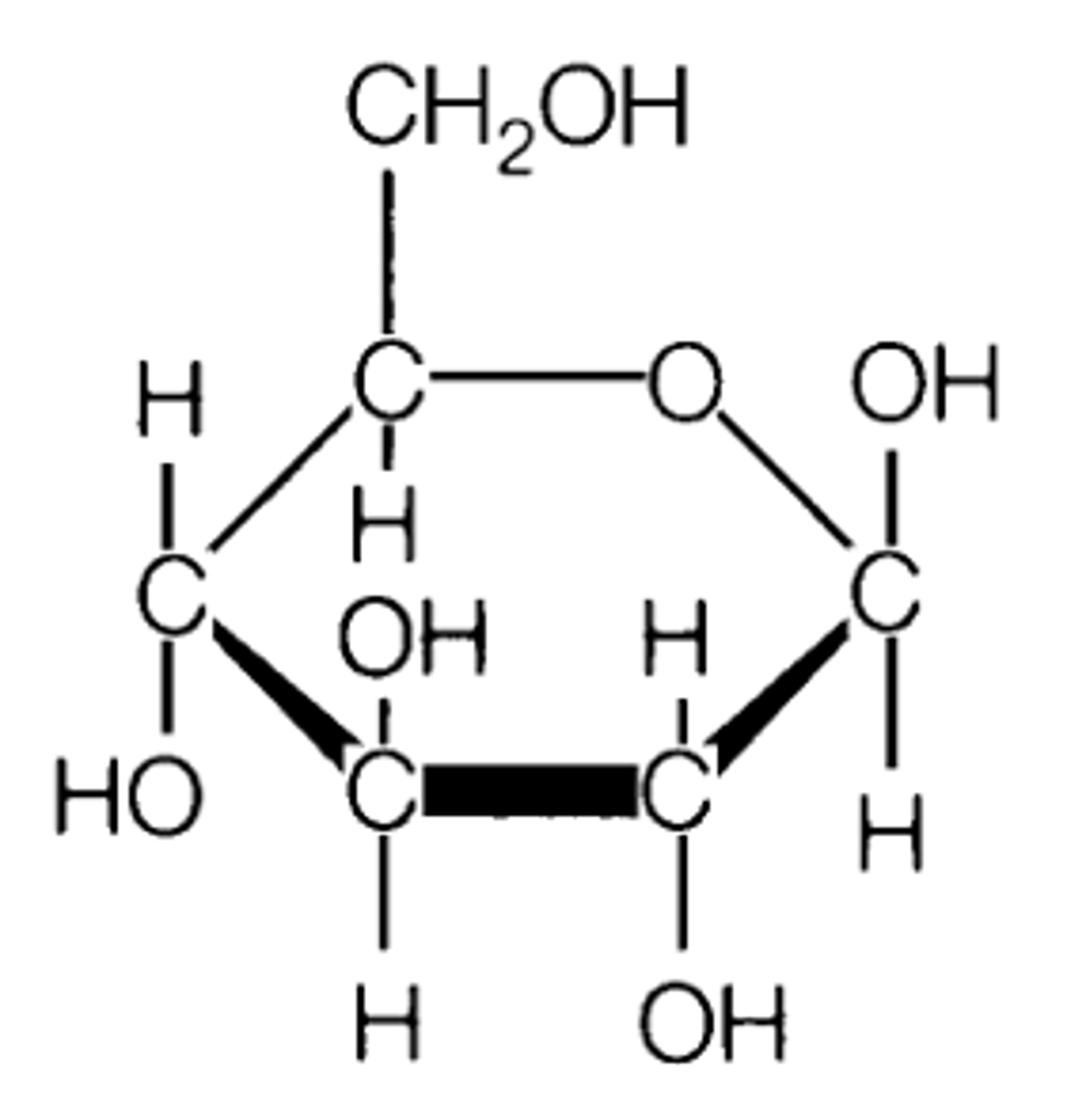
Carbohydrate is made up of
a carbonyl group (C=O), several Hydroxyl groups (-OH), along with multiple carbon-hydrogen bonds (C-H).
Monosaccharides
Simple sugars, the building blocks. Ex. glucose. If carbonyl groups or the number of carbon atoms present or the spatial arrangement of atoms is different, then the fucntion will be different.
Disaccarides
two monosaccharides joined by a covalent bond (glysosinic linkages). A dehydation reaction occurs.
Polysaccharides
a few hundred to thousands of monosaccharides formed by glysosinic linkages. They are usually used for storage excess energy and structure.
Carbs used for energy storage
Starch (plants) and glycogen (animals)
Carbs used for structural support
Cellulose (cell wall in plants), chitin (stiffens the wall of fungi), and peptidoglycan (cell wall in bacteria)
What do carbohydrates do?
They help in storage and structural support. As well as cell-cell recognition, and provides carbon molecules for more complex molecules.
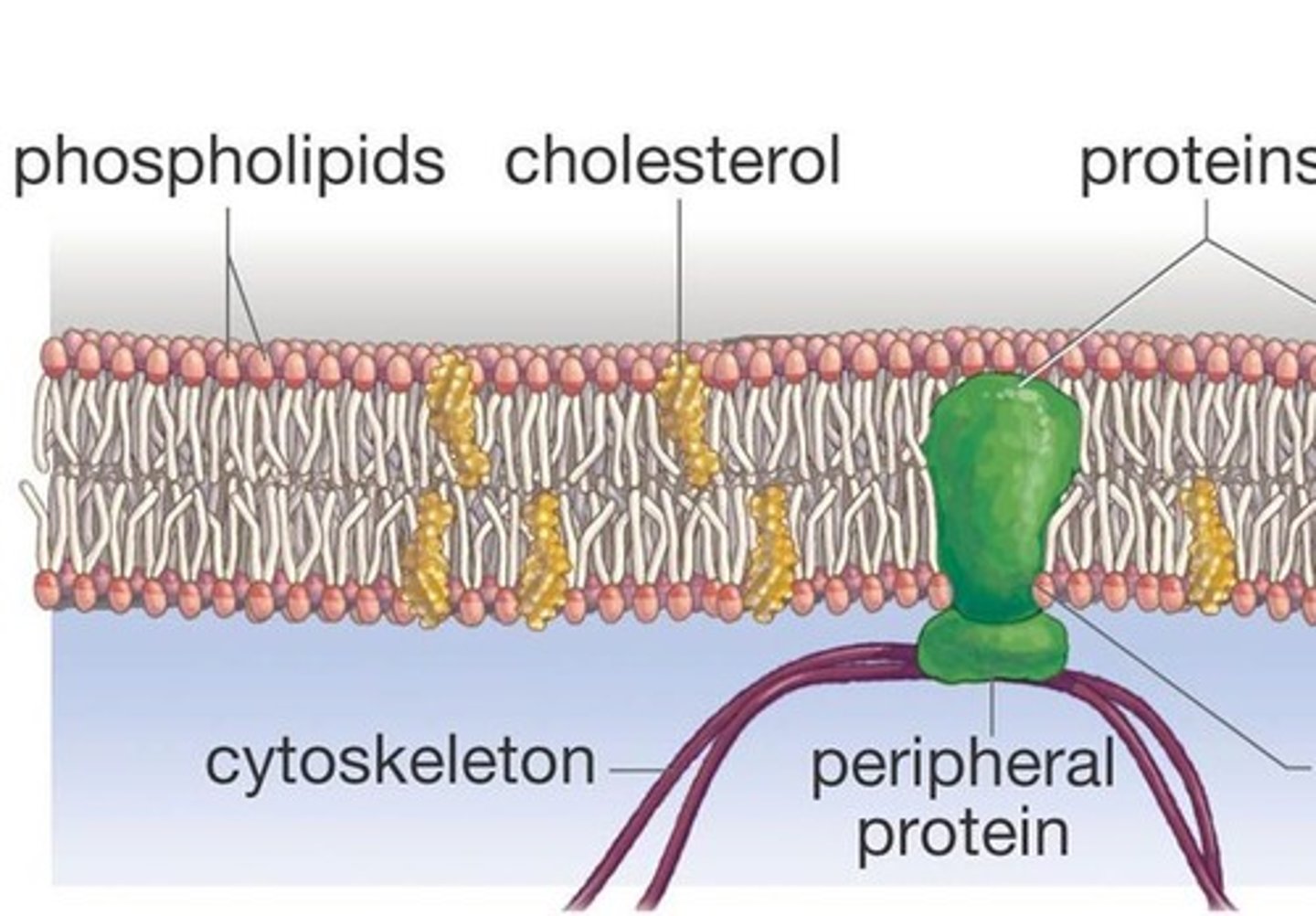
Lipids
Are hydrophobic so they don't mix with water. Most relevant types are steroids, fats, phospholipids.
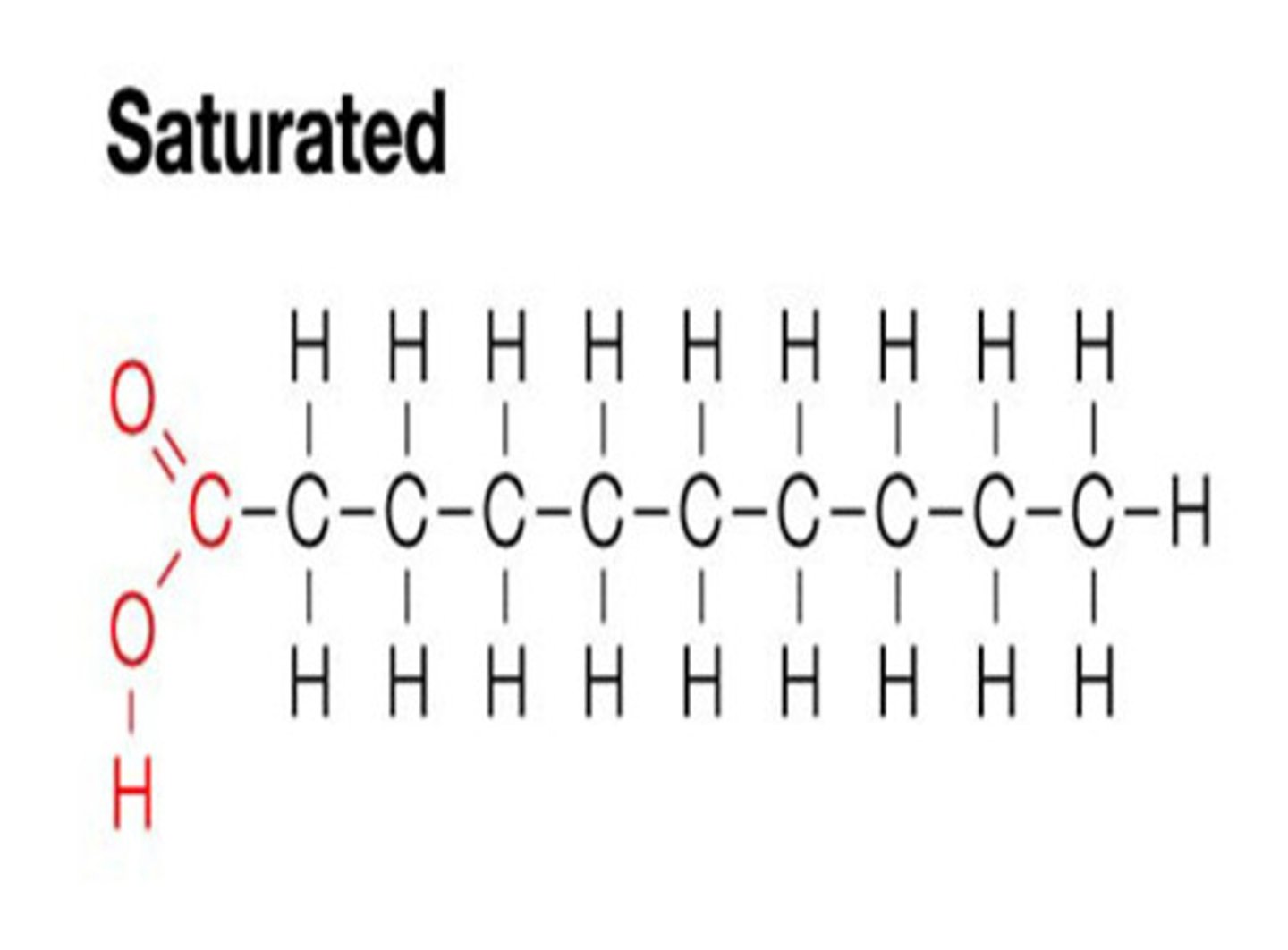
Saturated Fat
no double bonds between carbon atoms. Solid at room temperature because the structure can pack on itself and dense up.
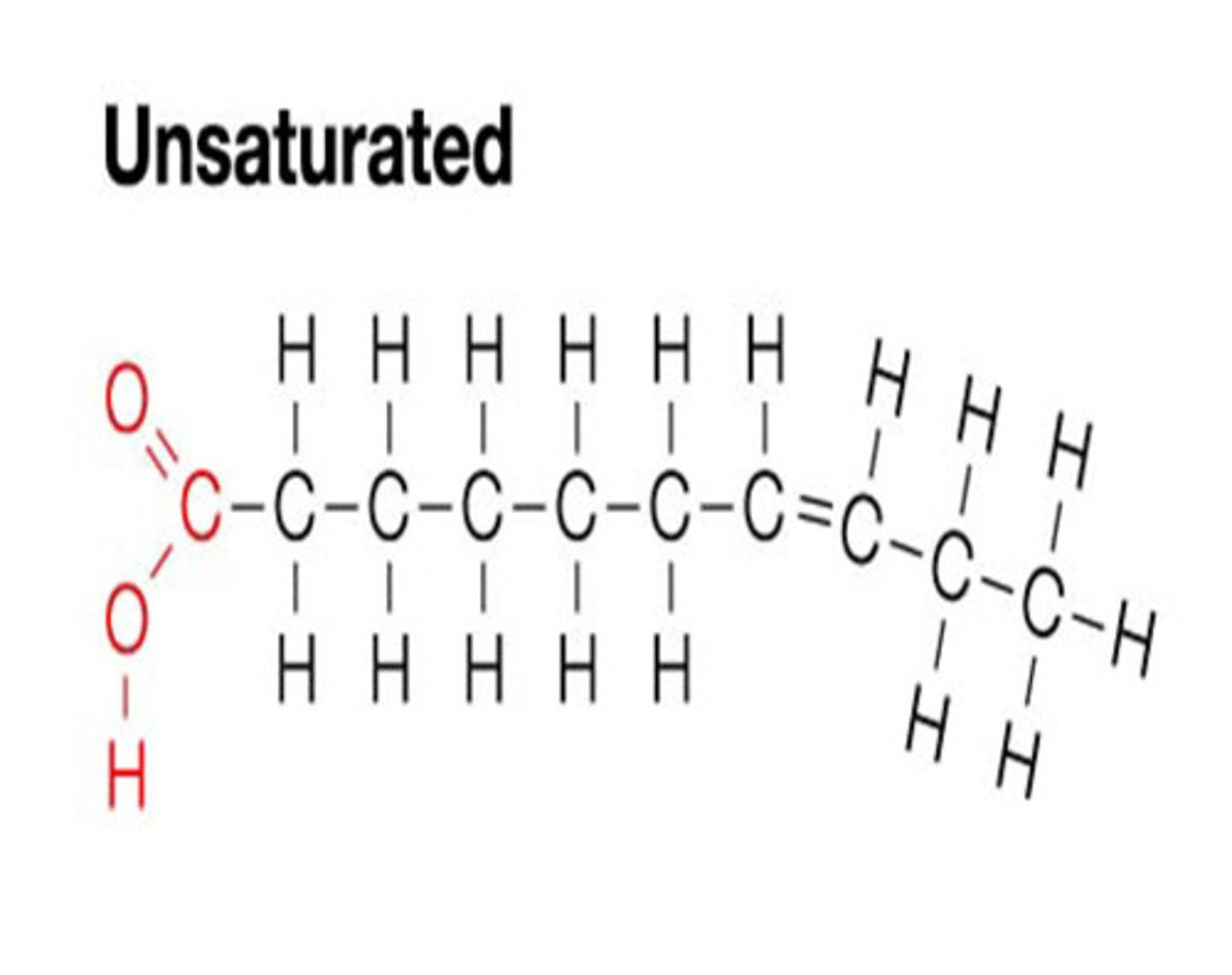
Unsaturated Fat
if one or more double bonds are present. The chain isnt completely straight. Liquid at room temperature.
Steroids
characterized with a four ringed structure.
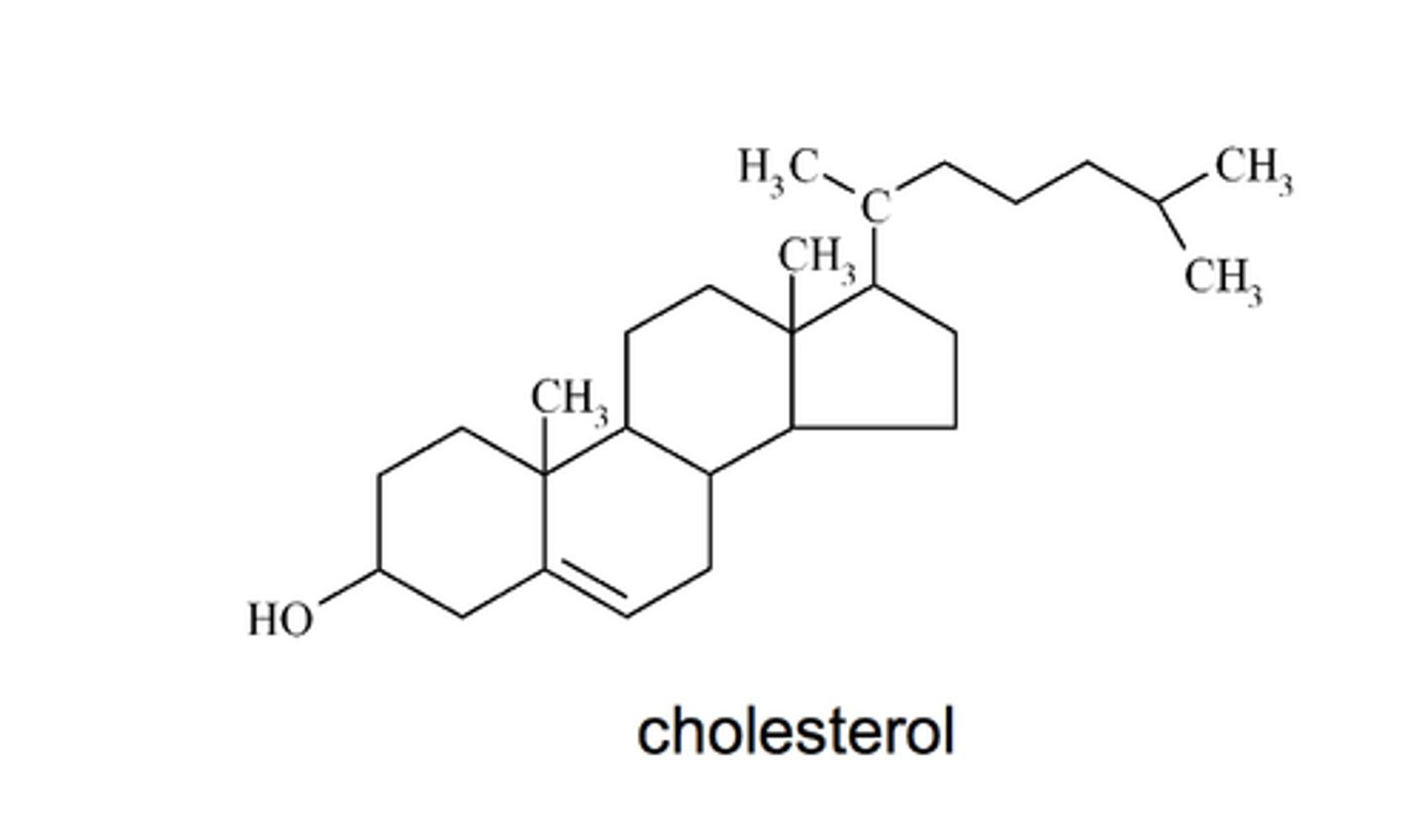
Cholesterol
important component of cell membranes. It is a steroid.
Fats
not actually a polymer. composed of three fatty acids that are linked to a three carbon molecule called glycerol. They are also called triglycerides.
Trans Fat
an unsaturated with added hydrogens to make the chain straight, most often man made.
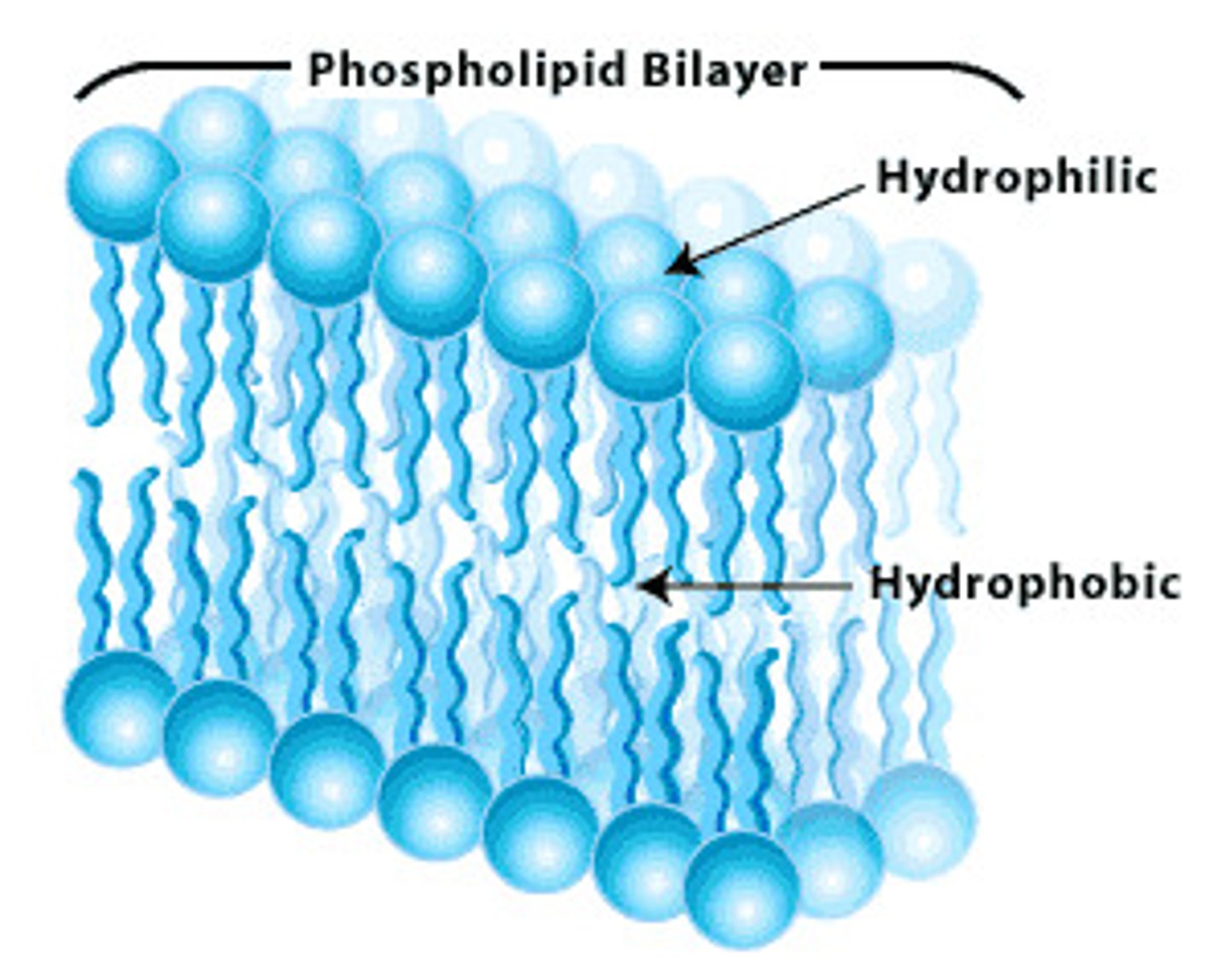
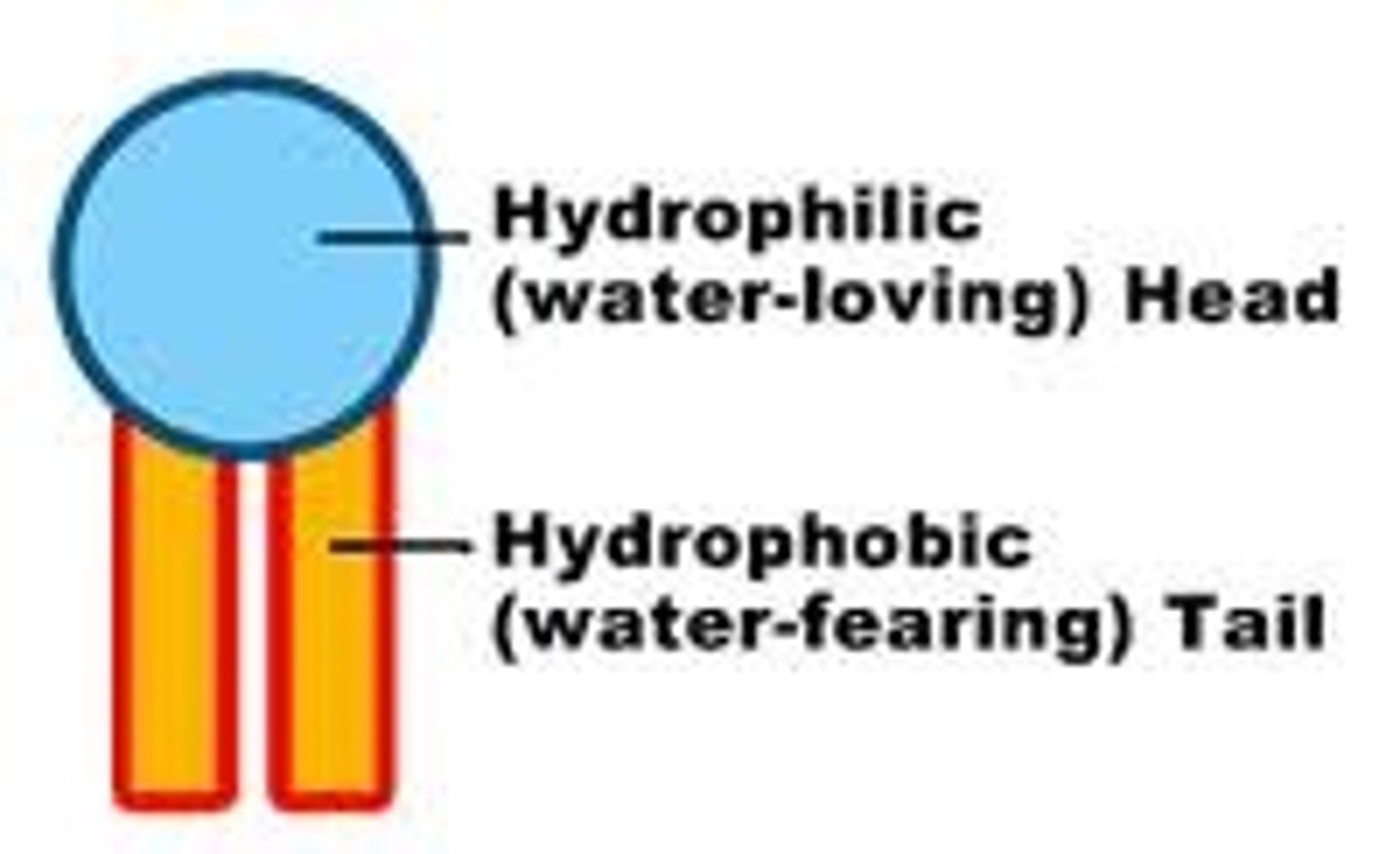
Phospholipids
Have a hydrophilic head and hydrophobic tail. In a bilayer, the heads point out and the tails point in.