Functional Groups // AP BIO
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Hydroxyl group
-OH
Hydrophilic, can form hydrogen bonds
increases solubility of compounds
alcohols

Carbonyl group
>C=O
Hydrophillic, can hydrogen bond, polar charges
Ketones, Aldehydes
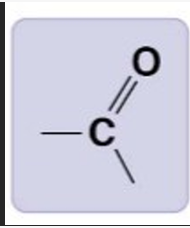
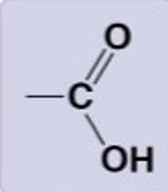
Carboxyl Group
-COOH
hydrophillic, highly polar, acidic, strong hydrogen bonds, donates protons
Carboxylic acids (organic acids)
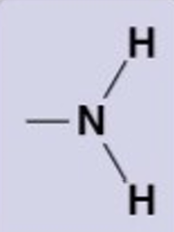
Amino group
NH2
hydrophillic, basic (accepts protons), forms h.b.
amino acids
Sulfhydryl group
-SH
Moderate hydrophillic, less polar than hydroxyl, forms disulfide bonds
proteins

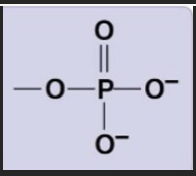
Phosphate group
-PO43-
hydrophillic, strongly polar, negative charge, water
ATP, neucleotides (DNA, RNA, nucleic acids)
Methyl group
-CH3
hydrophobic, nonpolar, cannot form hydrogen bonds, decreases solubility
methylated compounds
Functional group
groups that affect molecular function by being directly involved in chemical reactions
which group is always polar?
hydroxyl
which group determines the two groups of sugars?
carbonyl
which group has acidic properties?
carboxyl
which group acts as a base to attract protons?
amino
which group can form cross-links that stabilize protein structure?
sulfhydryl
which group is a component of atp?
phosphate
which group is nonpolar/ hydrophobic?
methyl
which group is a part of ethanol?
hydroxyl
which group is a part of ketones?
carbonyl
which group is a part of acetic acid?
carboxyl
which GROUPS are a part of glycine?
amino and carboxyl
which group is a part of cysteine?
sulfhydryl
glycerol
alcohol found in triglycerides