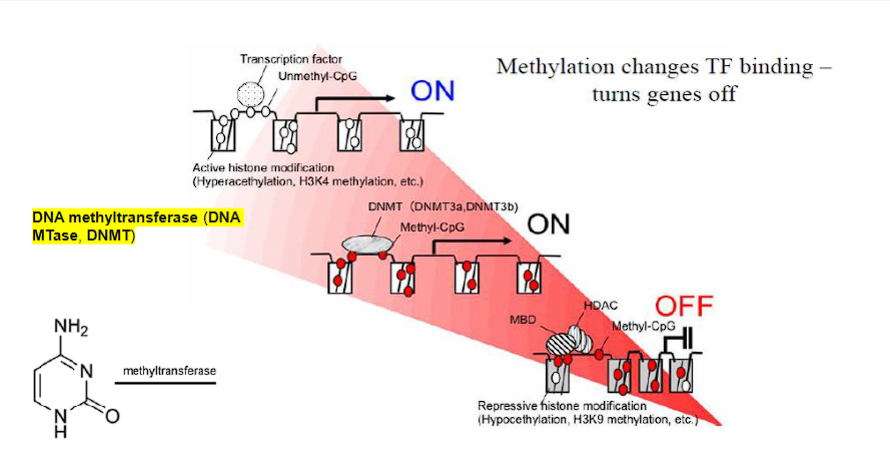
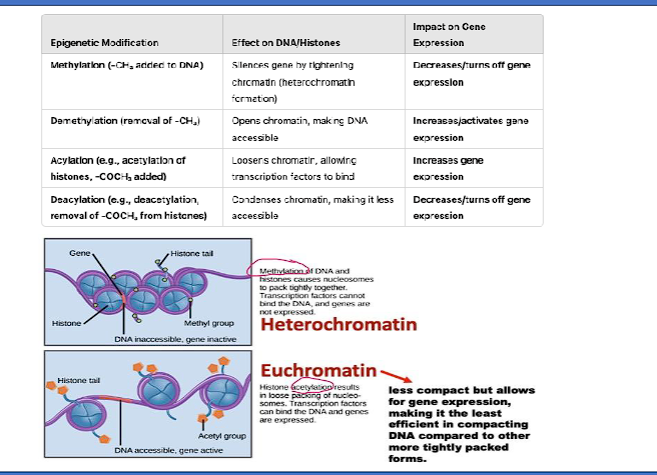
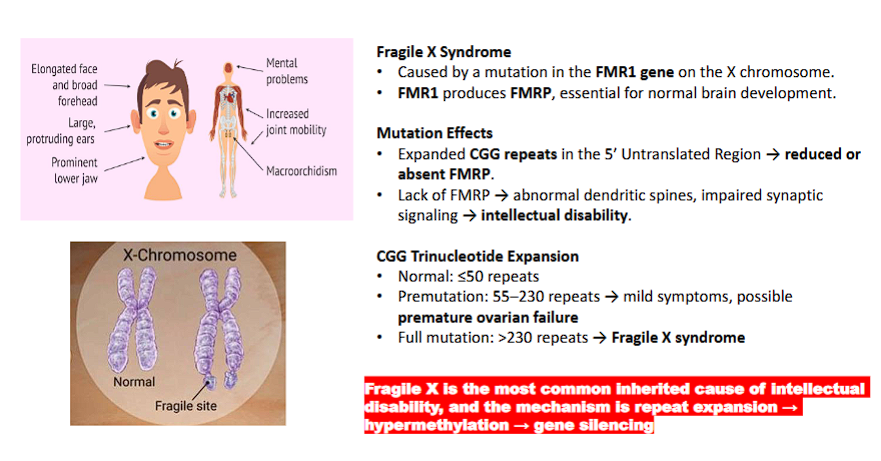
DNA - Dependent Production of RNA
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Describe the structure of gene regulatory elements and know the function of each: enhancer, silencer, promoter.
Explain the different kinds of RNA that are made and their roles in the cell.
Describe the central dogma of molecular biology, including how viruses have expanded the model.
Explain the different kinds of RNA that are made and their roles in the cell.
Explain the different types of transcriptional regulation and consider why some types of regulation are better than others.
List the steps of RNA synthesis and what must be accomplished in each step.
Compare the key differences between prokaryotic and eukaryotic transcription (gene organization/structure, location of specific events, families of promoters, coupling of translation).
clinical correlation
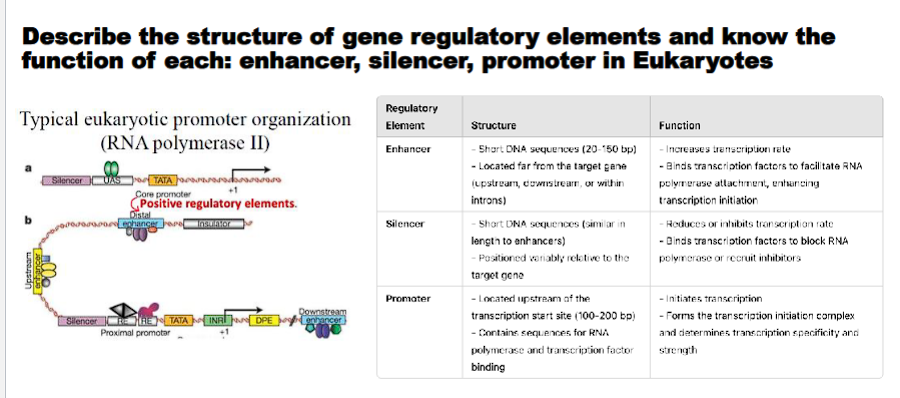
Describe the structure of gene regulatory elements and know the function of each: enhancer, silencer, promoter.
Gene regulatory elements are specific DNA sequences that control the transcription of a gene. They function by serving as binding sites for transcription factors and other proteins that influence RNA polymerase activity.
1. Promoter
The promoter is the foundational regulatory element that initiates transcription.
Function: To provide the binding site for RNA Polymerase II and the general transcription factors (GTFs). It defines the transcription start site (TSS) and the direction of transcription. It is essential for achieving a basal level of transcription.
Structure & Location:
Core Promoter: A region spanning approximately -50 to +50 base pairs relative to the Transcription Start Site (TSS). Key structural elements include:
TATA Box: A conserved AT-rich sequence (TATAAA) located about -25 to -30 bp upstream of the TSS. It is the binding site for the TATA-binding protein (TBP), a subunit of the general transcription factor TFIID, which helps recruit RNA Polymerase II.
Initiator (Inr): A sequence that overlaps the TSS itself (often with a consensus of YYANWYY).
Proximal Promoter: The region immediately upstream of the core promoter (within a few hundred base pairs). It contains binding sites for sequence-specific transcription factors that can enhance or repress the basal transcription level established by the core promoter.
Analogy: The promoter is like the keyhole and ignition switch of a car. It allows you to start the engine and get the car (RNA Polymerase) running at an idle (basal level).
2. Enhancer
Enhancers are elements that dramatically increase the rate of transcription of a gene.
Function: To enhance or boost transcription levels. They are binding platforms for activator proteins and co-activators. When bound, they help recruit RNA Polymerase II and the general transcription machinery to the promoter, leading to a significant increase in transcription.
Structure & Location:
Sequence: They are typically short (50-1500 bp), modular sequences that contain multiple binding sites (motifs) for different transcription factors.
Location: They are highly versatile in their location. They can be found:
Upstream of the promoter.
Downstream of the gene, within an intron.
Hundreds of kilobases away from the promoter.
Mechanism (The "Looping Model"): Despite their distance, enhancers are brought into physical proximity with the promoter through the looping of the DNA fiber. This looping is mediated by proteins called cohesins and is facilitated by the binding of activator proteins to the enhancer, which then interact with the mediator complex and proteins at the promoter.
Analogy: An enhancer is like a turbocharger. It's not part of the engine itself and can be located elsewhere in the car, but when activated, it connects to the engine to massively boost its power. The DNA loop is like the pipe connecting the turbo to the engine.
3. Silencer
Silencers are functional opposites of enhancers.
Function: To repress or reduce transcription levels. They are binding platforms for repressor proteins. When bound, they can inhibit transcription by preventing the assembly of the transcription machinery, recruiting chromatin-remodeling complexes that make DNA inaccessible, or directly interfering with activator proteins.
Structure & Location:
Sequence: Structurally, they are very similar to enhancers—they are also short, modular sequences with binding sites for specific proteins (in this case, repressors).
Location: Like enhancers, they can be located anywhere relative to the gene they control (upstream, downstream, or within introns).
Mechanism: Repressor binding can work in several ways:
Competitive Binding: The repressor binds to the same DNA site as an activator, blocking it.
Direct Interaction: The repressor binds to an activator protein and prevents its function.
Recruitment of Chromatin Modifiers: The repressor recruits histone deacetylases (HDACs) or other enzymes that condense chromatin, making the gene inaccessible.
Analogy: A silencer is like a parking boot. It can be clamped onto a wheel (any suitable location on the DNA) to completely prevent the car (transcription machinery) from moving, regardless of whether the ignition is on.
Summary Table
Element | Primary Function | Key Structural Features | Typical Location |
|---|---|---|---|
Promoter | Initiate basal transcription. Recruit RNA Polymerase II. | TATA Box, Initiator (Inr). Binding sites for general transcription factors. | Immediately upstream of the transcription start site (TSS). |
Enhancer | Boost transcription levels. | Modular clusters of binding sites for activator proteins. | Variable; can be upstream, downstream, within introns, or far away. |
Silencer | Repress transcription levels. | Modular clusters of binding sites for repressor proteins. | Variable; can be upstream, downstream, within introns, or far away. |
In summary, the promoter is the essential "on-switch" located at the gene's start, while enhancers and silencers are powerful, distant "dimmer switches" that fine-tune the level of gene expression up or down, respectively, through DNA looping and specific protein interactions.
what is a gene regulatory element?
how do gene regulatory elements work?
Gene regulatory elements are specific DNA sequences that control the transcription of a gene.
Gene regulatory elements serve as binding sites for transcription factors and other proteins that influence RNA polymerase activity.
Describe the structure of gene regulatory elements and know the function of each: enhancer, silencer, promoter.
promoter (one gene regulatory element): a promoter is dna sequence that promotes both the start of transcription and the direction of transcription.
RNA polymerase II AND general transcription factors bind to the promoter to start transcription.
example of a promoter: TATA box (conserved AT rich sequence)
Describe the structure of gene regulatory elements and know the function of each: enhancer, silencer, promoter.
enhancer: “enhance" the rate of a transcription of a gene.
binding platforms for activator proteins and co-activators.
when bound, the activator proteins and co-activators help recruit RNA polymerase II and the general transcription machinery to the promoter
thus, a significant increase in transcription is achieved.
Structure & Location:
Sequence: They are typically short (50-1500 bp), modular sequences that contain multiple binding sites (motifs) for different transcription factors.
Location: They are highly versatile in their location. They can be found:
Upstream of the promoter.
Downstream of the gene, within an intron.
Hundreds of kilobases away from the promoter.
Mechanism (The "Looping Model"): Despite their distance, enhancers are brought into physical proximity with the promoter through the looping of the DNA fiber. This looping is mediated by proteins called cohesins and is facilitated by the binding of activator proteins to the enhancer, which then interact with the mediator complex and proteins at the promoter.
Analogy: An enhancer is like a turbocharger. It's not part of the engine itself and can be located elsewhere in the car, but when activated, it connects to the engine to massively boost its power. The DNA loop is like the pipe connecting the turbo to the engine.
Describe the structure of gene regulatory elements and know the function of each: enhancer, silencer, promoter.
Silencers are functional opposites of enhancers.
Function: To repress or reduce transcription levels. They are binding platforms for repressor proteins. When bound, they can inhibit transcription by preventing the assembly of the transcription machinery, recruiting chromatin-remodeling complexes that make DNA inaccessible, or directly interfering with activator proteins.
Structure & Location:
Sequence: Structurally, they are very similar to enhancers—they are also short, modular sequences with binding sites for specific proteins (in this case, repressors).
Location: Like enhancers, they can be located anywhere relative to the gene they control (upstream, downstream, or within introns).
Mechanism: Repressor binding can work in several ways:
Competitive Binding: The repressor binds to the same DNA site as an activator, blocking it.
Direct Interaction: The repressor binds to an activator protein and prevents its function.
Recruitment of Chromatin Modifiers: The repressor recruits histone deacetylases (HDACs) or other enzymes that condense chromatin, making the gene inaccessible.
Analogy: A silencer is like a parking boot. It can be clamped onto a wheel (any suitable location on the DNA) to completely prevent the car (transcription machinery) from moving, regardless of whether the ignition is on.
what are the different ways repressing binding can work?
(competitive binding, direct interaction, recruitment of chromatin modifiers)
Repressor binding can work in several ways:
Competitive Binding: The repressor binds to the same DNA site as an activator, blocking it.
Direct Interaction: The repressor binds to an activator protein and prevents its function.
Recruitment of Chromatin Modifiers: The repressor recruits histone deacetylases (HDACs) or other enzymes that condense chromatin, making the gene inaccessible.
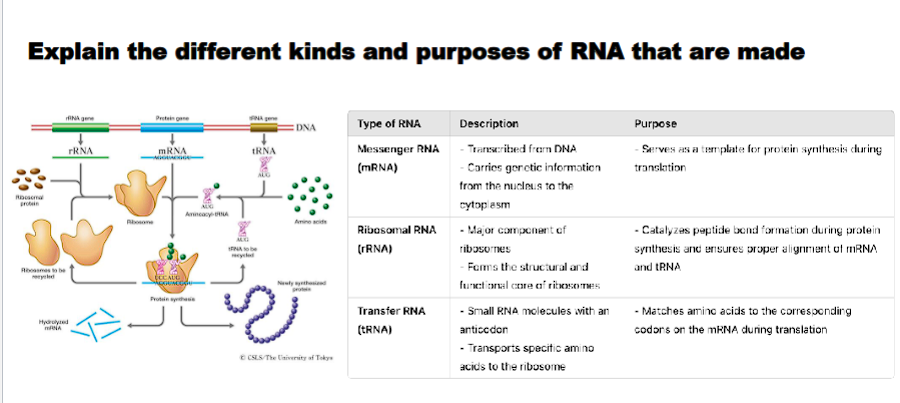
Explain the different kinds of RNA that are made and their roles in the cell
The central dogma of molecular biology (DNA → RNA → Protein) involves several types of RNA, each with a distinct structure and critical function. They are the versatile workhorses that execute the genetic instructions stored in DNA.
Here is an explanation of the different kinds of RNA, categorized by their primary function.
1. The Messenger: Protein-Coding RNAMessenger RNA (mRNA)
Role: Serves as the temporary genetic blueprint for protein synthesis. It carries the genetic code from the DNA in the nucleus to the ribosomes in the cytoplasm, where it is translated into a specific protein sequence.
Key Features:
Structure: It is a linear molecule that is complementary to a gene's DNA sequence.
Processing: In eukaryotes, pre-mRNA is extensively processed: a 5' cap is added, a 3' poly-A tail is added, and introns (non-coding regions) are spliced out. This creates the mature mRNA that is exported from the nucleus.
Lifecycle: It is short-lived, which allows the cell to rapidly adjust which proteins are made in response to changing conditions.
2. The Workbenches: Ribosomal RNARibosomal RNA (rRNA)
Role: The catalytic and structural core of the ribosome, the cell's protein-making factory. It is responsible for ribozyme activity, catalyzing the formation of peptide bonds between amino acids during translation.
Key Features:
Abundance: rRNA makes up about 60% of the ribosome's mass and is the most abundant type of RNA in a cell.
Structure: It folds into complex three-dimensional structures within the ribosome's large and small subunits.
Function: Provides the binding sites for mRNA and tRNA and directly catalyzes protein synthesis.
3. The Couriers: Transfer RNATransfer RNA (tRNA)
Role: Acts as a molecular adapter or "courier" that translates the language of mRNA (nucleotides) into the language of proteins (amino acids). Each tRNA molecule carries a specific amino acid to the growing polypeptide chain.
Key Features:
Structure: Has a characteristic cloverleaf secondary structure that folds into an L-shaped 3D structure.
Key Regions:
Anticodon: A sequence of three nucleotides that base-pairs with a complementary codon on the mRNA.
3' Acceptor End: The site where the specific amino acid is covalently attached.
Function: Ensures the correct amino acid is added according to the mRNA code.
4. The Regulators: Non-Coding RNAs
This is a large and diverse class of RNAs that are not translated into protein but play crucial regulatory roles.
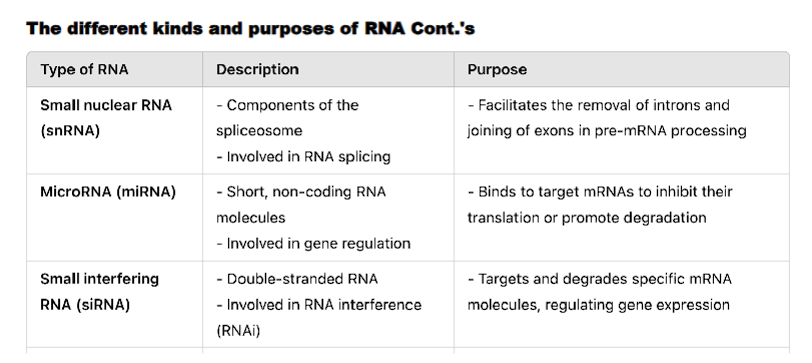
MicroRNA (miRNA) and Small Interfering RNA (siRNA)
Role: Key players in RNA interference (RNAi), a mechanism for silencing gene expression.
Function: They bind to complementary sequences on target mRNA molecules, typically leading to the degradation of the mRNA or blocking its translation. This fine-tunes gene expression and defends against viruses and transposons.
Small Nuclear RNA (snRNA)
Role: Essential components of the spliceosome, the large complex that removes introns from pre-mRNA in the nucleus.
Function: They recognize splice sites at the intron-exon boundaries and catalyze the splicing reaction.
Long Non-Coding RNA (lncRNA)
Role: A diverse class of RNAs longer than 200 nucleotides involved in epigenetic regulation.
Function: They can regulate gene expression at multiple levels by guiding chromatin-modifying complexes to specific genomic locations, influencing transcription, and affecting mRNA stability.
Summary Table
RNA Type | Full Name | Primary Role | Key Characteristic |
|---|---|---|---|
mRNA | Messenger RNA | Blueprint for protein synthesis. | Carries the genetic code from DNA to the ribosome. |
rRNA | Ribosomal RNA | Catalytic core of the ribosome. | Most abundant RNA; forms the ribosome's structure and catalyzes peptide bond formation. |
tRNA | Transfer RNA | Adapter in translation. | Brings the correct amino acid to the ribosome based on the mRNA codon. |
miRNA/siRNA | MicroRNA / Small Interfering RNA | Post-transcriptional gene silencing. | Binds to mRNA to inhibit translation or trigger its degradation. |
snRNA | Small Nuclear RNA | Pre-mRNA splicing. | Forms the core of the spliceosome complex. |
lncRNA | Long Non-Coding RNA | Epigenetic and transcriptional regulation. | Regulates gene expression by interacting with DNA, RNA, and proteins. |
In essence, while DNA is the stable master archive of genetic information, the various types of RNA are the active managers and workers that interpret, copy, regulate, and execute the instructions to build and maintain the cell.
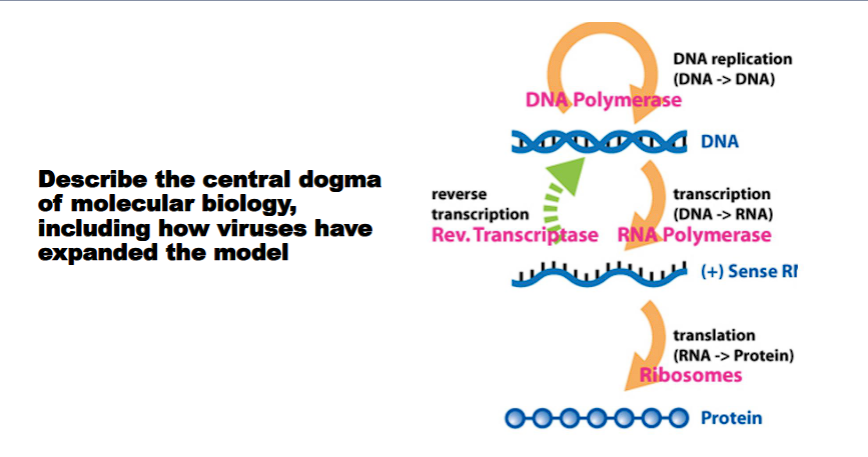
Describe the central dogma of molecular biology, including how viruses have expanded the model.
The Central Dogma of Molecular Biology is a fundamental framework that describes the flow of genetic information within a biological system. It was first articulated by Francis Crick in 1958.
The Central Dogma states that genetic information flows in a specific, sequential direction:
DNA → RNA → Protein
This unidirectional flow can be broken down into three key processes:
Replication: DNA is copied to DNA. This ensures that genetic information is passed faithfully from one generation of cells to the next.
Transcription: DNA is copied into RNA. In this process, the genetic instructions in a gene are rewritten into a messenger RNA (mRNA) molecule.
Translation: RNA is used to build a protein. The mRNA sequence is "read" by a ribosome, which assembles a corresponding sequence of amino acids into a functional protein.
The following flowchart illustrates this core information pathway, along with the key processes involved at each stage:
How Viruses Have Expanded the Model
Viruses, which lack their own replication machinery and must hijack a host cell's, have been found to violate the strict unidirectionality of the original Central Dogma. They have revealed that information flow can follow other paths, as shown in the expanded diagram below.
The key exceptions discovered are:
1. RNA → DNA (Reverse Transcription)
This process, which goes "backwards" from the original dogma, was discovered in retroviruses (e.g., HIV).
Mechanism: These viruses have an RNA genome. When they infect a cell, they use a viral enzyme called reverse transcriptase to copy their RNA genome into DNA.
Significance: This viral DNA is then integrated into the host's own genome, where it can be transcribed into new viral RNA and proteins. This pathway (RNA → DNA) fundamentally expanded the Central Dogma.
2. RNA → RNA (RNA Replication)
Many viruses have RNA as their genetic material and do not use DNA at any point in their life cycle.
Mechanism: These viruses (e.g., SARS-CoV-2, Influenza virus) encode an RNA-dependent RNA polymerase. This enzyme uses an RNA strand as a template to synthesize a complementary RNA strand, which is then used to make new viral particles.
Significance: This established that genetic information could be replicated and transmitted directly from RNA to RNA, without needing a DNA intermediate.
Summary Table: The Flows of Genetic Information
Process | Description | Example |
|---|---|---|
DNA → DNA | Replication. Standard copying of the genome. | All cellular life (bacteria, plants, animals). |
DNA → RNA | Transcription. Creation of an RNA copy of a gene. | All cellular life. |
RNA → Protein | Translation. Synthesis of a protein based on an RNA code. | All cellular life. |
RNA → DNA | Reverse Transcription. An RNA template is used to make DNA. | Retroviruses (e.g., HIV). |
RNA → RNA | RNA Replication. An RNA template is used to make RNA. | RNA viruses (e.g., Coronavirus, Picornavirus). |
The Universal Rule: Protein → ?
One direction is notably absent and is believed to be impossible: information never flows from protein back to nucleic acid (Protein → DNA/RNA). The sequence of a protein cannot be used to recreate or alter the genetic code that specified it. This is what makes the Central Dogma a "dogma"—it is a foundational, one-way principle upon which molecular biology is built, even with the expansions provided by viruses.
In conclusion, the Central Dogma remains the core model for information flow in cells. Viruses have not disproven it but have instead expanded our understanding of the possible pathways genetic information can take in the biological world.
what is the central dogma, and what three key processes is it broken down into?
The Central Dogma states that genetic information flows in a specific, sequential direction:
DNA → RNA → Protein
This unidirectional flow can be broken down into three key processes:
Replication: DNA is copied to DNA. This ensures that genetic information is passed faithfully from one generation of cells to the next.
Transcription: DNA is copied into RNA. In this process, the genetic instructions in a gene are rewritten into a messenger RNA (mRNA) molecule.
Translation: RNA is used to build a protein. The mRNA sequence is "read" by a ribosome, which assembles a corresponding sequence of amino acids into a functional protein.
how have viruses expanded the model?
viruses use reverse transcription (RNA→ DNA).
the viral rna undergoes reverse transcription to become viral dna (opposite of the central dogma)
OR many viruses just go from RNA to RNA and don’t use DNA at all.
viruses have been found to violate the strict unidirectionality of the original Central Dogma. They have revealed that information flow can follow other paths, as shown in the expanded diagram below.
the viral rna undergoes reverse transcription to become viral dna (opposite of the central dogma)
OR many viruses just go from RNA to RNA and don’t use DNA at all.
what is the mechanism of viral infection?
the virus enters the host cell
the virus has its OWN RNA Genome
the viral enzyme reverse transcriptase uses the viruses’s own RNA as a template to create cDNA (complementary DNA).
this cDNA is used to make a double stranded viral DNA molecule.
the viral dsDNA is transported into the host’s cells nucleus and integrated into the host’s chromosomal DNA.
it’s now called a provirus
the genetic material of a virus as incorporated into, and able to replicate with, the genome of a host cell.
Transcription and Translation. The host cell's machinery (RNA Polymerase II, ribosomes, etc.) is now hijacked. It treats the integrated proviral DNA like any other gene in its own genome:
The host transcribes the proviral DNA into new viral RNA molecules.
These new viral RNA molecules serve two purposes:
a) They act as mRNA to be translated by the host's ribosomes into viral proteins (like the capsid and reverse transcriptase).
b) They become the genomes for new virus particles.
Assembly and Release. The new viral RNA genomes and viral proteins assemble into new virus particles that bud from the host cell to infect others.
what are the different types of mRNA?
mRNA
rRNA
tRNA
miRNA
siRNA
snRNA
IncRNA
The universe of RNA is vast and functional, extending far beyond its classic role as a mere messenger. Here is a detailed breakdown of the different kinds of RNA and their critical roles in the cell, categorized by their primary function.
Overview of Major RNA Types
RNA Type | Full Name | Primary Role | Key Characteristic |
|---|---|---|---|
mRNA | Messenger RNA | Blueprint for protein synthesis. | Carries the genetic code from DNA to the ribosome. |
rRNA | Ribosomal RNA | Catalytic core & scaffold of the ribosome. | Most abundant RNA; forms the ribosome's structure and catalyzes peptide bond formation. |
tRNA | Transfer RNA | Adapter in translation. | Brings the correct amino acid to the ribosome based on the mRNA codon. |
miRNA | MicroRNA | Post-transcriptional gene silencing. | Fine-tunes gene expression by repressing translation or triggering mRNA decay. |
siRNA | Small Interfering RNA | Post-transcriptional gene silencing & defense. | Defends against viruses and transposons by guiding mRNA cleavage. |
snRNA | Small Nuclear RNA | Pre-mRNA splicing. | Forms the core of the spliceosome complex. |
lncRNA | Long Non-Coding RNA | Epigenetic & transcriptional regulation. | Regulates gene expression by interacting with DNA, RNA, and proteins. |
Other | (e.g., snoRNA, piRNA) | Nucleotide modification, transposon silencing. | Specialized "housekeeping" and regulatory functions. |
Detailed Roles and Functions1. The Protein-Coding Messenger: mRNA
Role: Serves as the temporary genetic blueprint for protein synthesis. It carries the genetic code from the DNA in the nucleus to the ribosomes in the cytoplasm.
Key Features:
Structure: In eukaryotes, it is extensively processed with a 5' cap, 3' poly-A tail, and splicing to remove introns, creating mature mRNA.
Lifecycle: It is short-lived, allowing the cell to rapidly adjust protein production in response to changing conditions.
2. The Ribosomal Machinery: rRNA
Role: The structural and catalytic foundation of the ribosome. It is a ribozyme, meaning the RNA itself catalyzes the formation of peptide bonds during translation.
Key Features:
Abundance: Makes up about 60% of the ribosome's mass and is the most abundant RNA in a cell.
Function: Provides binding sites for mRNA and tRNA and directly catalyzes protein synthesis.
3. The Molecular Adapter: tRNA
Role: Acts as a bilingual "courier molecule." It translates the language of nucleic acids (mRNA codons) into the language of proteins (amino acids).
Key Features:
Structure: Has a characteristic cloverleaf secondary structure that folds into an L-shaped 3D structure.
Key Regions:
Anticodon: Base-pairs with the complementary mRNA codon.
3' Acceptor End: The site where the corresponding amino acid is covalently attached.
4. The Gene Regulators: Non-Coding RNAs (ncRNAs)
This is a large and diverse class of RNAs that are not translated into protein but are essential for regulating gene expression.
MicroRNA (miRNA) and Small Interfering RNA (siRNA):
Role: Key players in RNA interference (RNAi), a sequence-specific silencing pathway.
Mechanism: They guide a protein complex (RISC) to complementary mRNA molecules, leading to translational repression or mRNA degradation.
Difference: miRNAs are encoded by the genome and fine-tune endogenous gene expression, while siRNAs often originate from viruses or double-stranded RNA and act in defense.
Small Nuclear RNA (snRNA):
Role: Essential components of the spliceosome.
Function: They recognize intron-exon boundaries and catalyze the splicing of pre-mRNA in the nucleus.
Long Non-Coding RNA (lncRNA):
Role: A diverse class of RNAs (>200 nucleotides) involved in epigenetic regulation.
Function: They can silence or activate genes by guiding chromatin-modifying complexes to specific genomic locations, influencing transcription, and affecting mRNA stability.
Other Specialized ncRNAs:
snoRNA (Small Nucleolar RNA): Guides the chemical modification (e.g., methylation, pseudouridylation) of rRNA and other RNAs.
piRNA (Piwi-interacting RNA): Primarily found in the germline, where they silence transposable elements to protect genomic integrity.
In essence, while DNA is the stable master archive of genetic information, the various types of RNA are the active managers and workers. They interpret, copy, regulate, and execute the instructions to build and maintain the cell, demonstrating that RNA is far more than just a passive messenger.
types of transcriptional regulation
types of transcriptional regulation and then discuss why some are considered more advantageous than others in specific contexts.
The Central Dogma and the Importance of Transcription Control
Transcription is the first step in gene expression, where DNA is copied into messenger RNA (mRNA). Regulating this step is crucial for a cell because it is:
Energy Efficient: It prevents the wasteful production of unnecessary RNA and subsequent proteins.
Responsive: It allows the cell to quickly adapt to changing environmental conditions.
Deterministic: It controls cell identity, development, and complex processes by turning the right genes on and off at the right time.
Types of Transcriptional Regulation
Transcriptional regulation can be categorized in several ways. The most common and functionally significant classification is based on the effect of a regulatory molecule (like a protein or RNA) on the transcription process.
1. Positive Regulation (Activation)
In positive regulation, transcription is increased by the presence of a specific regulatory molecule called an activator.
Mechanism: An activator protein binds to a specific DNA sequence (e.g., an enhancer or an upstream activator sequence). This binding helps recruit RNA polymerase to the promoter, often with the help of co-activator proteins, leading to an increase in the rate of transcription initiation.
Analogy: This is like hiring a motivational speaker (the activator) to encourage a worker (RNA polymerase) to start a project.
Classic Example: The CAP (Catabolite Activator Protein) in the lac operon of E. coli. When glucose is absent and lactose is present, cAMP levels rise. cAMP binds to CAP, which then binds to the lac promoter and dramatically enhances RNA polymerase's ability to transcribe the lactose-digestion genes.
2. Negative Regulation (Repression)
In negative regulation, transcription is decreased by the presence of a specific regulatory molecule called a repressor.
Mechanism: A repressor protein binds to a specific DNA sequence (e.g., an operator) and physically blocks RNA polymerase from accessing the promoter or moving along the DNA, thereby preventing transcription.
Analogy: This is like a security guard (the repressor) locking the door to an office, preventing the worker (RNA polymerase) from entering.
Classic Example: The Lac Repressor in the same lac operon. In the absence of lactose, the repressor binds to the operator and shuts down transcription. When lactose is present, it binds to the repressor, changing its shape and causing it to fall off the DNA, allowing transcription to proceed.
Crucial Point: These two types are not mutually exclusive. A single gene or operon can be under both positive and negative control, allowing for very precise and complex regulation (as seen in the lac operon).
Why Are Some Types of Regulation "Better" Than Others?
The "better" type of regulation depends entirely on the biological context and the default state the cell needs for a particular gene. There is no universally "best" type, but each has distinct strategic advantages.
Let's compare them based on key principles:
1. Default State and Energy Efficiency
Negative Regulation is optimal for genes that are needed frequently (Housekeeping genes).
Default State = ON. The gene is transcribed unless actively turned off by a repressor.
Why it's "better" here: It's energetically cheaper to produce a single repressor protein to turn off a few unnecessary genes than to produce multiple activators to turn on hundreds of essential genes. For example, genes for core metabolic processes are usually constitutively expressed and only repressed under specific conditions.
Positive Regulation is optimal for genes that are rarely needed.
Default State = OFF. The gene is silent unless actively turned on by an activator.
Why it's "better" here: It prevents wasteful "leaky" expression. The cell ensures that energetically expensive pathways (e.g., digesting a rare sugar) are only activated when the specific signal (the activator) is present.
2. Response Time and Safety
Negative Regulation can lead to faster activation.
To turn a gene ON, the cell simply needs to remove the repressor. This can be a very fast process (e.g., through allosteric inactivation). This is ideal for genes that need to be rapidly deployed in response to a changing environment.
Positive Regulation can be safer for potentially harmful genes.
If a gene's product could be damaging if expressed at the wrong time (e.g., a toxin or a powerful developmental signal), keeping it "off by default" is a fail-safe mechanism. It requires a positive, specific signal to be activated, reducing the risk of accidental expression.
3. Regulatory Complexity and Integration
Positive Regulation is superior for integrating multiple signals.
A single gene promoter can have binding sites for multiple different activators. Transcription can be triggered by activator A OR activator B, or it can require activator A AND activator B. This combinatorial control is a cornerstone of complex eukaryotic gene regulation, allowing a finite number of transcription factors to generate immense diversity in gene expression patterns for development and cellular differentiation.
Summary Table
Feature | Positive Regulation (Activation) | Negative Regulation (Repression) |
|---|---|---|
Mechanism | Activator protein enhances transcription. | Repressor protein blocks transcription. |
Default State | OFF (Gene is silent without activator). | ON (Gene is expressed without repressor). |
Energy Efficiency | Better for genes rarely needed. | Better for genes frequently needed. |
Response to Signal | Signal induces transcription. | Signal derepresses transcription. |
Safety | Fail-safe. Prevents accidental expression. | Fast activation. Quick response by removing a block. |
Complexity | Excellent for combinatorial control. | Less common for complex integration. |
Conclusion
No single type of transcriptional regulation is inherently "better." Instead, they are complementary tools used by the cell to solve different problems.
Use Negative Regulation when you want a gene on most of the time and need to turn it off quickly and efficiently.
Use Positive Regulation when you want a gene off most of the time, need a fail-safe, or want to integrate multiple signals.
The sophisticated interplay between activators and repressors, often regulating the same gene, is what allows for the precise and dynamic control of gene expression that is essential for all life.
types of transcriptional regulation (positive regulation)
activator
1. Positive Regulation (Activation): transcription is increased by the presence of a specific regulatory molecule called an activator.
Mechanism: An activator protein binds to a specific DNA sequence (e.g., an enhancer or an upstream activator sequence). This binding helps recruit RNA polymerase to the promoter, often with the help of co-activator proteins, leading to an increase in the rate of transcription initiation.
Analogy: This is like hiring a motivational speaker (the activator) to encourage a worker (RNA polymerase) to start a project.
Classic Example: The CAP (Catabolite Activator Protein) in the lac operon of E. coli. When glucose is absent and lactose is present, cAMP levels rise. cAMP binds to CAP, which then binds to the lac promoter and dramatically enhances RNA polymerase's ability to transcribe the lactose-digestion genes.
types of transcriptional regulation (negative regulation)
repressor
2. Negative Regulation (Repression)
transcription is decreased by the presence of a specific regulatory molecule called a repressor.
Mechanism: A repressor protein binds to a specific DNA sequence (e.g., an operator) and physically blocks RNA polymerase from accessing the promoter or moving along the DNA, thereby preventing transcription.
Analogy: This is like a security guard (the repressor) locking the door to an office, preventing the worker (RNA polymerase) from entering.
Classic Example: The Lac Repressor in the same lac operon. In the absence of lactose, the repressor binds to the operator and shuts down transcription. When lactose is present, it binds to the repressor, changing its shape and causing it to fall off the DNA, allowing transcription to proceed.
Crucial Point: These two types are not mutually exclusive. A single gene or operon can be under both positive and negative control, allowing for very precise and complex regulation (as seen in the lac operon).
what is negative regulation optimal for?
Negative Regulation is optimal for genes that are needed frequently (Housekeeping genes).
Default State = ON. The gene is transcribed unless actively turned off by a repressor.
Why it's "better" here: It's energetically cheaper to produce a single repressor protein to turn off a few unnecessary genes than to produce multiple activators to turn on hundreds of essential genes. For example, genes for core metabolic processes are usually constitutively expressed and only repressed under specific conditions.
what is positive regulation optimal for?
Positive Regulation is optimal for genes that are rarely needed.
Default State = OFF. The gene is silent unless actively turned on by an activator.
Why it's "better" here: It prevents wasteful "leaky" expression. The cell ensures that energetically expensive pathways (e.g., digesting a rare sugar) are only activated when the specific signal (the activator) is present.
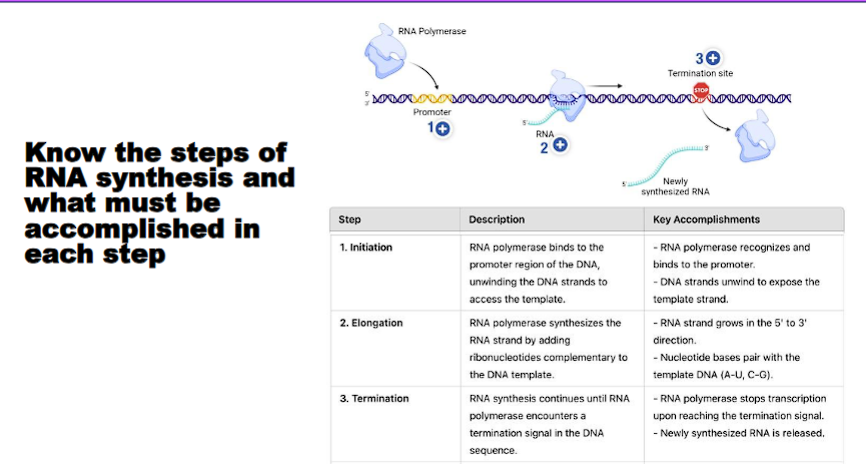
Know the steps of RNA synthesis and what must be accomplished in each step
Here is a detailed breakdown of the steps of RNA synthesis, specifically for transcription in prokaryotes, with notes on key eukaryotic differences.
Transcription is the process of copying a segment of DNA into a complementary RNA strand. It is carried out by the enzyme RNA polymerase. The process is divided into three main stages: Initiation, Elongation, and Termination.
Stage 1: Initiation
Objective: To correctly position RNA polymerase at the start of a gene and unwind the DNA to form the transcription bubble.
Steps and Key Accomplishments:
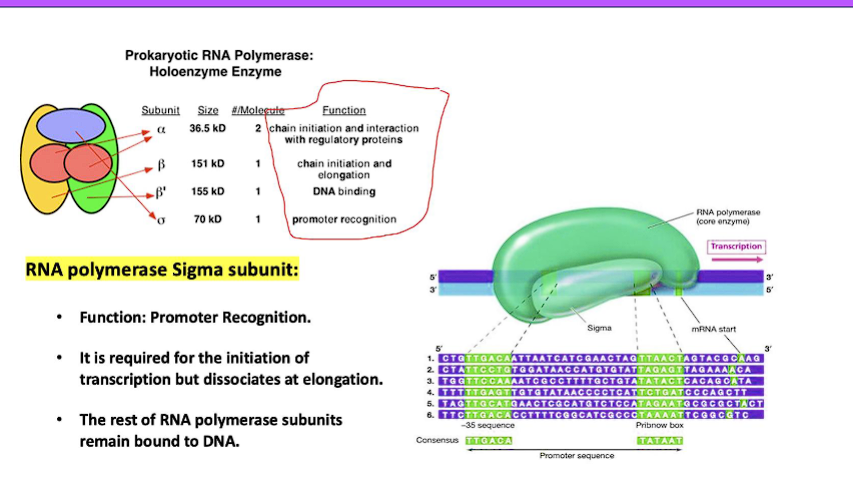
Promoter Recognition:
What happens: RNA polymerase binds to a specific DNA sequence called the promoter, which is located upstream of the gene to be transcribed.
What is accomplished: This ensures that transcription begins at the correct location. In prokaryotes, the sigma (σ) factor subunit of RNA polymerase is essential for promoter recognition. In eukaryotes, a collection of transcription factors (TFs) first bind to the promoter (e.g., TATA box), which then helps recruit RNA polymerase II.
Formation of the Closed Complex:
What happens: The RNA polymerase and associated factors bind to the promoter while the DNA remains double-stranded.
What is accomplished: The enzyme is correctly positioned and poised to begin transcription.
Formation of the Open Complex:
What happens: Approximately 14 base pairs of DNA at the transcription start site are unwound, separating the two strands.
What is accomplished: This creates the "transcription bubble," providing the enzyme access to the template strand.
First Nucleotide Addition:
What happens: The first complementary ribonucleoside triphosphate (rNTP—ATP, GTP, CTP, or UTP) aligns with the +1 start site on the template strand. The second rNTP is brought in, and the first phosphodiester bond is formed.
What is accomplished: The RNA chain is officially initiated. After this, in prokaryotes, the sigma factor often dissociates, and the core enzyme proceeds to the next stage.
Stage 2: Elongation
Objective: To sequentially add nucleotides to the growing RNA chain, moving along the DNA template.
Steps and Key Accomplishments:
Template-Directed Synthesis:
What happens: RNA polymerase moves along the template strand in the 3' → 5' direction. For each DNA base on the template strand, it recruits the complementary rNTP (A, U, G, C).
What is accomplished: The RNA strand is synthesized in the 5' → 3' direction, complementary to the template strand and identical (with U for T) to the coding/non-template strand.
Catalysis of Phosphodiester Bonds:
What happens: The 3' hydroxyl (OH) group of the last nucleotide in the chain attacks the high-energy phosphate of the incoming rNTP, releasing pyrophosphate (PPi). This reaction is catalyzed by RNA polymerase.
What is accomplished: The RNA chain is elongated by one nucleotide, and the energy for the reaction is provided by the hydrolysis of the rNTP.
Unwinding and Rewinding of DNA:
What happens: As RNA polymerase moves forward, it continuously unwinds the DNA ahead of the catalytic site (downstream) and rewinds the DNA behind it (upstream).
What is accomplished: The transcription bubble (~17 base pairs) moves with the enzyme, maintaining a single-stranded region for the template.
Proofreading (to a limited extent):
What happens: RNA polymerase has a rudimentary proofreading ability. If an incorrect nucleotide is incorporated, the enzyme can back up and cleave the mispaired nucleotide.
What is accomplished: This reduces the error rate, though it is much less effective than DNA polymerase's proofreading. The consequences of errors in RNA are less severe than in DNA.
Stage 3: Termination
Objective: To recognize the end of the gene, release the completed RNA transcript, and dissociate RNA polymerase from the DNA.
There are two primary mechanisms in prokaryotes:
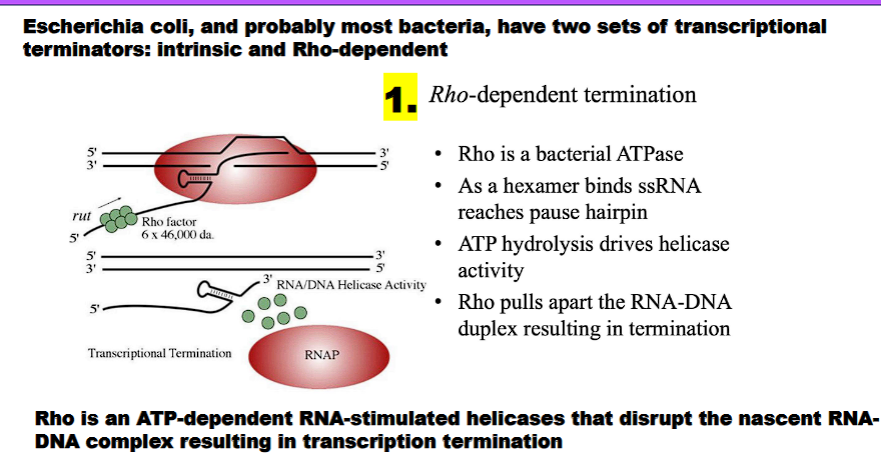
A. Rho (ρ)-Dependent Termination
Pause Site:
What happens: RNA polymerase transcribes a region that causes it to pause.
What is accomplished: This pause gives the Rho protein time to catch up to the polymerase.
Rho Factor Binding and Translocation:
What happens: The Rho factor, a helicase protein, binds to a specific sequence on the RNA (the rut site) and uses ATP to translocate along the RNA towards the transcription bubble.
What is accomplished: Rho "chases" the paused RNA polymerase.
Helicase Activity and Release:
What happens: Upon reaching the transcription bubble, Rho's helicase activity unwinds the RNA-DNA hybrid within the polymerase.
What is accomplished: This forces the release of the RNA transcript and the dissociation of RNA polymerase from the DNA.
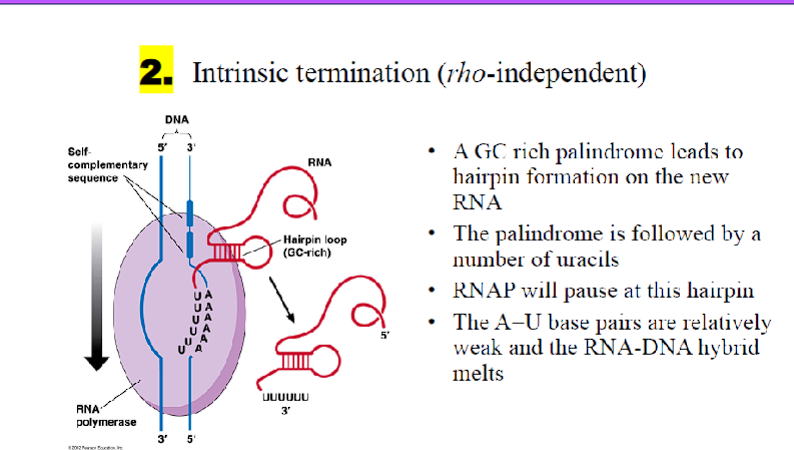
B. Rho (ρ)-Independent Termination (Intrinsic Termination)
GC-Rich Hairpin Formation:
What happens: The RNA transcript itself contains a self-complementary sequence, which forms a stable stem-loop structure (hairpin) just upstream of the termination point.
What is accomplished: The formation of this hairpin causes RNA polymerase to pause and destabilizes its grip on the transcript.
Poly-U Tract:
What happens: The hairpin is immediately followed by a sequence of uracils (U) in the RNA, which base-pair weakly with a sequence of adenines (A) in the DNA template.
What is accomplished: The weak A-U bonds are not strong enough to hold the RNA-DNA hybrid together. The paused polymerase stalls, and the RNA transcript dissociates, terminating transcription.
Key Differences in Eukaryotes:
Initiation: Requires many transcription factors (TFs) to form a pre-initiation complex before RNA Polymerase II can bind.
Modification: The primary RNA transcript (pre-mRNA) is not functional immediately. It must be processed:
5' Capping: A modified guanine nucleotide is added to the 5' end. This protects the RNA and is recognized by the ribosome.
3' Polyadenylation: A tail of adenine nucleotides (poly-A tail) is added to the 3' end. This aids in export from the nucleus and stability.
RNA Splicing: Non-coding sequences (introns) are removed, and coding sequences (exons) are joined together.
Termination: The process is coupled with the cleavage and polyadenylation of the pre-mRNA. RNA Polymerase II continues transcribing well past the poly-A signal before terminating.
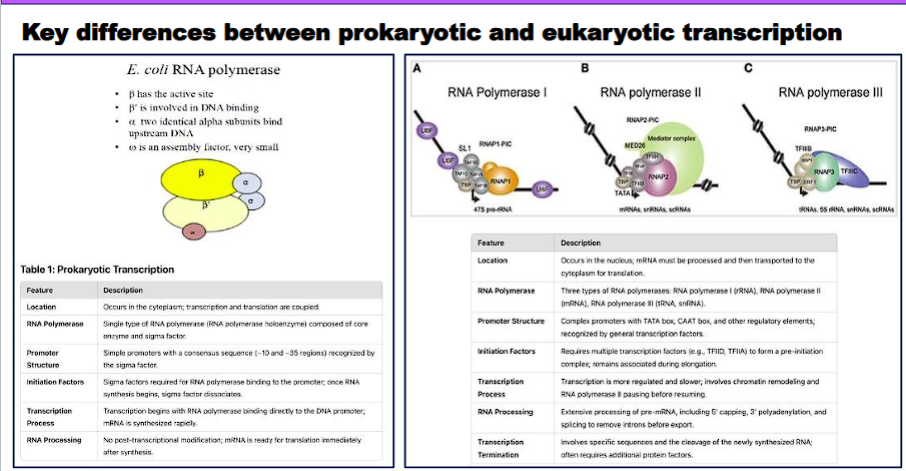
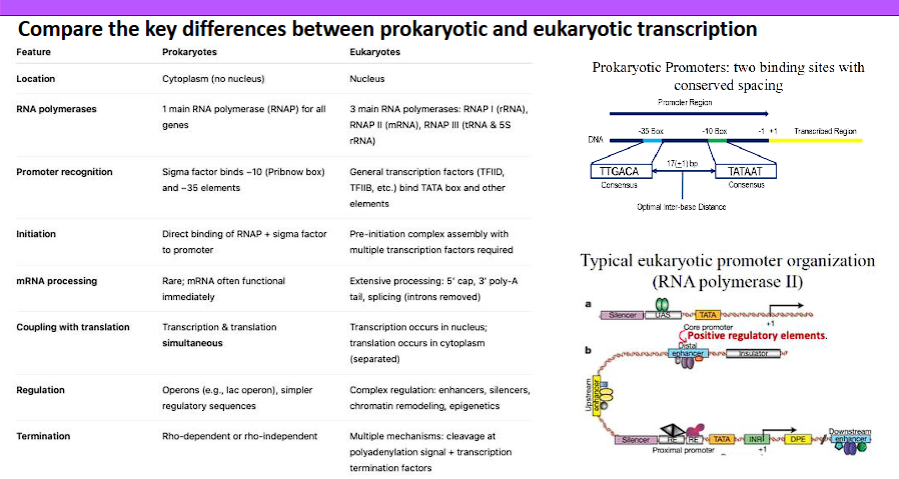
Compare the key differences between prokaryotic and eukaryotic transcription (gene organization/structure, location of specific events, families of promoters, coupling of translation).
Here is a detailed comparison of the key differences between prokaryotic and eukaryotic transcription, structured for clarity.
Overview
The fundamental process of transcription (DNA → RNA) is conserved, but the complexity of eukaryotic cells, with their nucleus and elaborate regulation, leads to profound differences in gene organization, machinery, and processing. Prokaryotic transcription is simpler and more efficient, while eukaryotic transcription is more complex and highly regulated.
Comparison Table
Feature | Prokaryotes | Eukaryotes |
|---|---|---|
1. Gene Organization & Structure | Operons: Multiple related genes (e.g., for a metabolic pathway) are transcribed together as a single polycistronic mRNA. | Monocistronic Genes: Each gene is transcribed individually from its own promoter. One mRNA = one protein. |
2. Introns & Exons | Virtually absent. Genes are continuous coding sequences. The primary transcript is the mature mRNA. | Very common. Genes contain non-coding introns and coding exons. The primary transcript (pre-mRNA) requires splicing. |
3. Location of Events | Cytoplasm. Transcription and translation are coupled; translation begins on the mRNA while it is still being synthesized. | Compartmentalized. Transcription occurs in the nucleus. Translation occurs in the cytoplasm. They are spatially and temporally separated. |
4. RNA Polymerase | A single type of RNA polymerase (with multiple sigma (σ) factors to recognize different promoters). | Three main types: |
5. Promoters | Simple and conserved. Two key sequences: the -10 box (Pribnow box: TATAAT) and the -35 box (TTGACA). Recognized directly by the σ factor. | Complex and diverse. |
6. Initiation Complex | Simple. RNA polymerase core enzyme + σ factor = Holoenzyme. It binds directly to the promoter. | Highly Complex. Requires the assembly of many Transcription Factors (TFs) before RNA Polymerase II can bind: |
7. RNA Processing | Minimal to none. The mRNA is typically used immediately for translation without modification. | Extensive and essential. The pre-mRNA undergoes three major processing steps in the nucleus: |
8. Coupling of Translation | Yes, tightly coupled. Translation begins while the mRNA is still being transcribed. There is no nuclear envelope to separate the processes. | No coupling. Transcription is complete, and the mRNA is fully processed and exported from the nucleus before translation can begin in the cytoplasm. |
Key Differences Explained1. Gene Organization: Operons vs. Monocistronic Genes
Prokaryotes (Operons): This is an efficient strategy for a simple cell. By grouping genes for a single pathway (like the lac operon for lactose metabolism), the cell can coordinately regulate their expression with one promoter, turning the entire pathway on or off as a single unit.
Eukaryotes (Monocistronic): This allows for more sophisticated, individual regulation of each gene, which is necessary for the complex development and cell differentiation seen in multicellular organisms.
2. Compartmentalization: Location and Coupling
Prokaryotes: The lack of a nucleus means the entire process is open. The coupling of transcription and translation provides extreme speed and efficiency. As soon as the 5' end of an mRNA emerges from RNA polymerase, a ribosome can bind and start translating it.
Eukaryotes: The nuclear envelope physically separates transcription from translation. This allows for a critical quality control step: the mRNA must be fully processed (capped, spliced, polyadenylated) before it is exported to the cytoplasm. This prevents the translation of defective or immature mRNA molecules.
3. Promoter and Initiation Complexity
Prokaryotes: Initiation is straightforward. The sigma factor is the key that unlocks promoter recognition. Different sigma factors allow the cell to respond to global changes (e.g., heat shock, nitrogen starvation) by activating different sets of genes.
Eukaryotes: Initiation is a major point of regulation. The need for a large Pre-Initiation Complex and the existence of distant enhancers allows a vast number of regulatory inputs (from various transcription factors activated by different signals) to be integrated at a single promoter. This combinatorial control is fundamental to eukaryotic complexity.
4. RNA Processing: The Defining Eukaryotic Feature
Prokaryotes: mRNA is "ready-to-use."
Eukaryotes: Processing is not just an add-on; it is essential for creating a functional mRNA and provides additional layers of regulation:
The 5' cap and 3' poly-A tail protect the mRNA from degradation and are recognized by the translation machinery.
Splicing allows for alternative splicing, where a single gene can be spliced in different ways to produce multiple related but distinct proteins. This dramatically increases the proteomic diversity from a limited number of genes.
Summary
Aspect | Prokaryotic Transcription | Eukaryotic Transcription |
|---|---|---|
Core Principle | Speed & Efficiency for a single-celled organism. | Complexity & Regulation for a multicellular organism. |
Defining Feature | Coupling of transcription and translation. | Compartmentalization and extensive RNA processing. |
Regulatory Strategy | Simple on/off switches via operons and sigma factors. | Layered, combinatorial control via transcription factors and RNA processing |