Biology Test - October
1/62
Earn XP
Description and Tags
Stuff
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
Carbohydrates are s_____ s_____. These have 1 or two sugar molecules.
Simple sugars
Lipids are c______ s_ugars. These contain 3 or more sugar molecules.
Complex sugars
Nucleic Acids are ___
DNA
Proteins are made by _________
Ribosomes
The Big Three atoms for life are H______, O_____ and C_____
Hydrogen, Oxygen and Carbon
Two more important atoms for life are p__________ and ________
Phosphorous and Nitrogen
A hydroxyl group is made of a single bonded O_____ and _______
Oxygen and hydrogen
A carboxyl is made of a double bonded oxygen and carbon (a carbonyl group), along with a second ______ and _______
Oxygen and hydrogen
A carbonyl group is made of a double bonded ______ and ______
Oxygen and carbon
An amino group is made of a ________ single bonded to two ________ atoms
Answer format: ________, ________
Nitrogen, hydrogen
A phosphate group is made of a phosphate double bonded to an ______ and single bonded to 3 other ______s and two ________ atoms
Answer format: ______, ______s, ________
Oxygen, oxygens, hydrogen
A methyl group is defined at __3
Answer Format: __3 (both letters are capitals)
CH3
Carbohydrates contain C_____, H_______ and ______
Carbon, Hydrogen and Oxygen
Carbohydrates are the main source of cellular ______
Energy
Carbohydrates typically end in - ___
OSE
Carbohydrates refers to ______ + h_______ (water)
Answer format: ______, ________
Carbon, hydrates
Cellulose, a POLY-saccharide, is found in P_____ and is used for _________
Answer format: P_____, _________
Plants, structure
Starch, a POLY-saccharide, is found in P_____ and is used for _______ in roots
Answer format: P_____, _________
Plants, storage
Glycogen, a POLY-saccharide, is found in A______ and is used for _______ (in humans in liver and muscles)
Answer format: A______, _______
Animals, storage
To break or make a Di-saccharide or a POLY-saccharide you must add or remove H2_. This processes is known as Dehydration synthesis for making a bond and hydrolysis for breaking a bond.
H2O
Proteins are made of the elements C_____, H_______ and ______
Carbon, Hydrogen and Oxygen
Examples of the monomers of Carbohydrates include F_______, G______ and Ga_______
Fructose, Glucose and Galactose
Examples of the Polymers of Carbohydrates are C_______, S_____ and G_______
Cellulose, Starch and Glycogen
Proteins are made of the elements ______, H______ and O_____
Carbon, Hydrogen and Oxygen
The monomers of Protiens are A____ A____ and there are 20 different types
Amino Acids
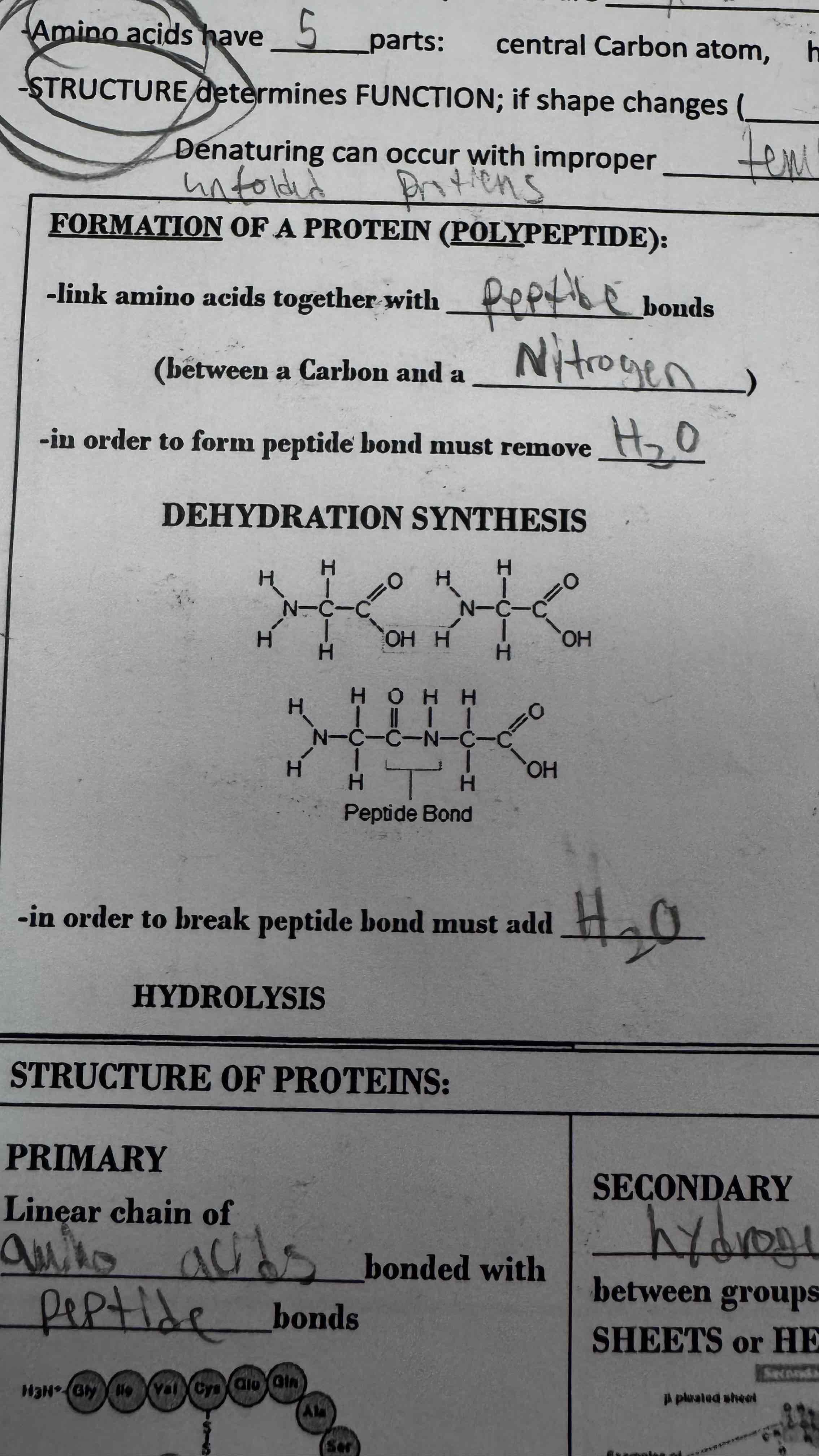
Amino Acids are linked together with this type of bond (do not include the word bond in response)
Peptide
A peptide bond is a bond between a C_____ & Nitrogen
Carbon & Nitrogen
To break or make a peptide bond, you must add or subtract _2_
H2O
You DO NOT get energy from protiens
Retype this
You DO NOT get energy from proteins
Protiens are used for S________, Support/Movement and S______ just to name a few
Answer format: St_______, ________
Structure, Storage
Amino acids contain _ parts: central carbon atom, hydrogen, Amino group, Carboxyl group and an “R group”
5
Shape changing = being _________. This occurs when a protein is subjected to improper temperature and PH (acidity)
Denatured
Protein polymers include: elastin, collagen, plant Protiens and protein peptides
Retype this
Protein polymers include: elastin, collagen, plant Protiens and protein peptides
An E_____ is a type of protein that can be reused over and over,
Enzyme
Need examples of proteins and all of the stuff for lipids. Wth happened to my flashcards!
Uses for proteins include structure, storage, antibodies and more. Retype this
Uses for proteins include structure, storage, antibodies and more. Retype this
The primary structure of a protein is a linear chain of amino acids bonded with peptide
The primary structure of a protein is a linear chain of amino acids bonded with peptide
The secondary structure of proteins are made of hydrogen bonds between groups = pleated sheets or helix
The secondary structure of proteins are made of hydrogen bonds between groups = pleated sheets or helix
The tertiary structure of proteins are additional bonds/interactions which form a globular shape
The tertiary structure of proteins are additional bonds/interactions which form a globular shape
The quaternary structure of a protein is made of more than only polypeptide chain(s)
The quaternary structure of a protein is made of more than only polypeptide chain(s)
Lipids are composed of C, H, O
Carbon, Hydrogen, Oxygen
Lipids are a secondary source of energy, major part of the membrane of cells
Lipids are a secondary source of energy, major part of the membrane of cells
lipids are water hating / _________
Hydrophobic
Triglycerides are fats and oils. They are made of a glycerol chain and 3 fatty acid chains
Triglycerides are fats and oils. They are made of a glycerol chain and 3 fatty acid chains
Phospholipids have a phosphate containing head that loves water and fatty acid tails that hate water
Phospholipids have a phosphate containing head that loves water and fatty acid tails that hate water
Waxes are a type of lipid that are regularly found in nature and repel water. Examples include earwax, beeswax and the coatings of leaves, fruits and feathers.
Waxes are a type of lipid that are regularly found in nature and repel water. Examples include earwax, beeswax and the coatings of leaves, fruits and feathers.
Steroids are a type of lipid are are used to construct hormones.
Steroids are a type of lipid are are used to construct hormones.
An unsaturated fat is a type of triglyceride which is a lipid. These fats are mostly plant based, are liquid at room temperature and contain some double bonds between carbons
An unsaturated fat is a type of triglyceride which is a lipid. These fats are mostly plant based, are liquid at room temperature and contain some double bonds between carbons
Saturated fats are triglycerides and therefore lipids. These were mostly animal fats, are solid at room temperature and contain no double bonds between carbons.
Saturated fats are triglycerides and therefore lipids. These were mostly animal fats, are solid at room temperature and contain no double bonds between carbons.
To make a bond dehydration synthesis is needed whereas to break a bond hydrolysis is required.
To make a bond dehydration synthesis is needed whereas to break a bond hydrolysis is required.
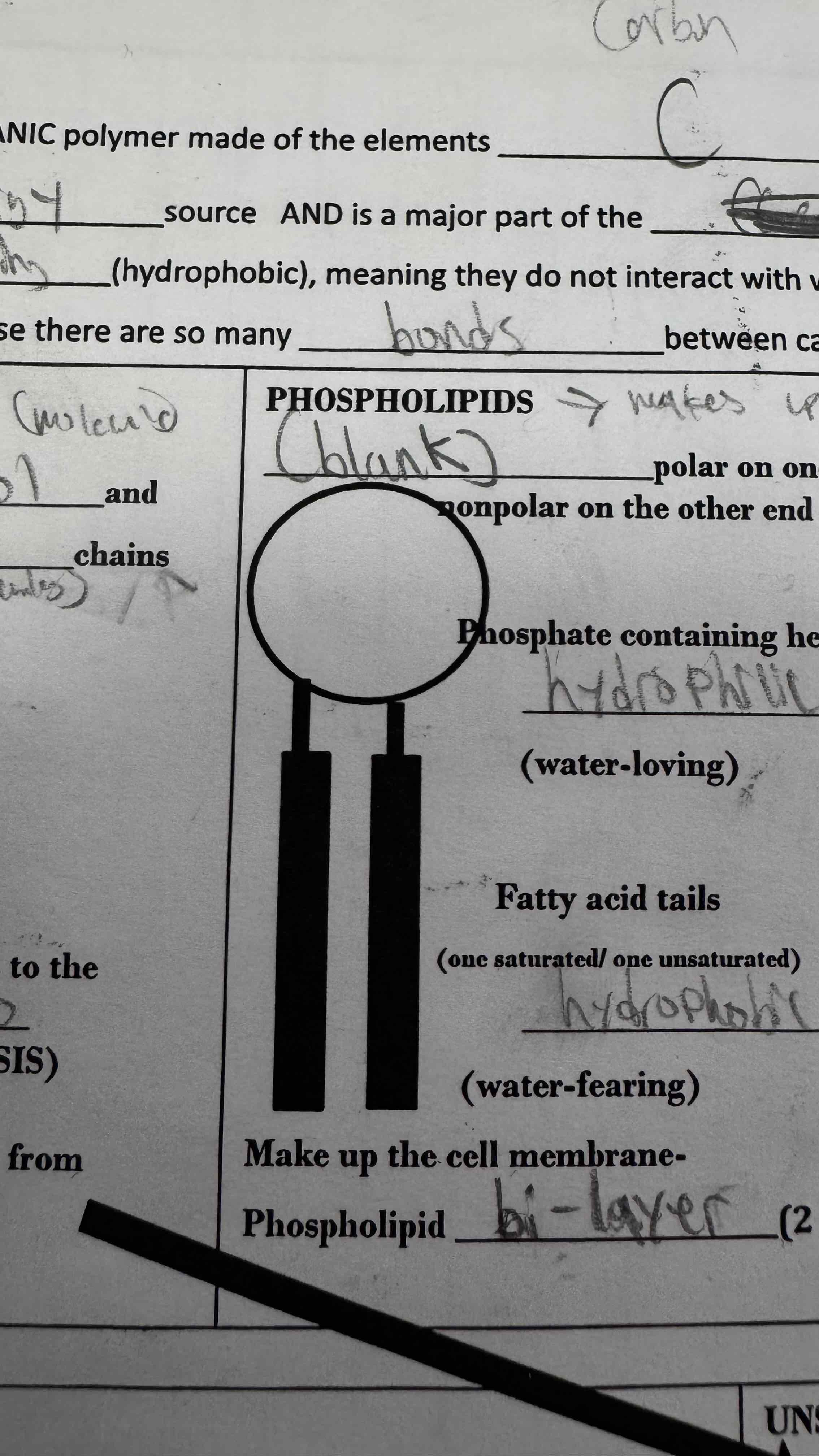
What type of lipid is this?
Phospholipid
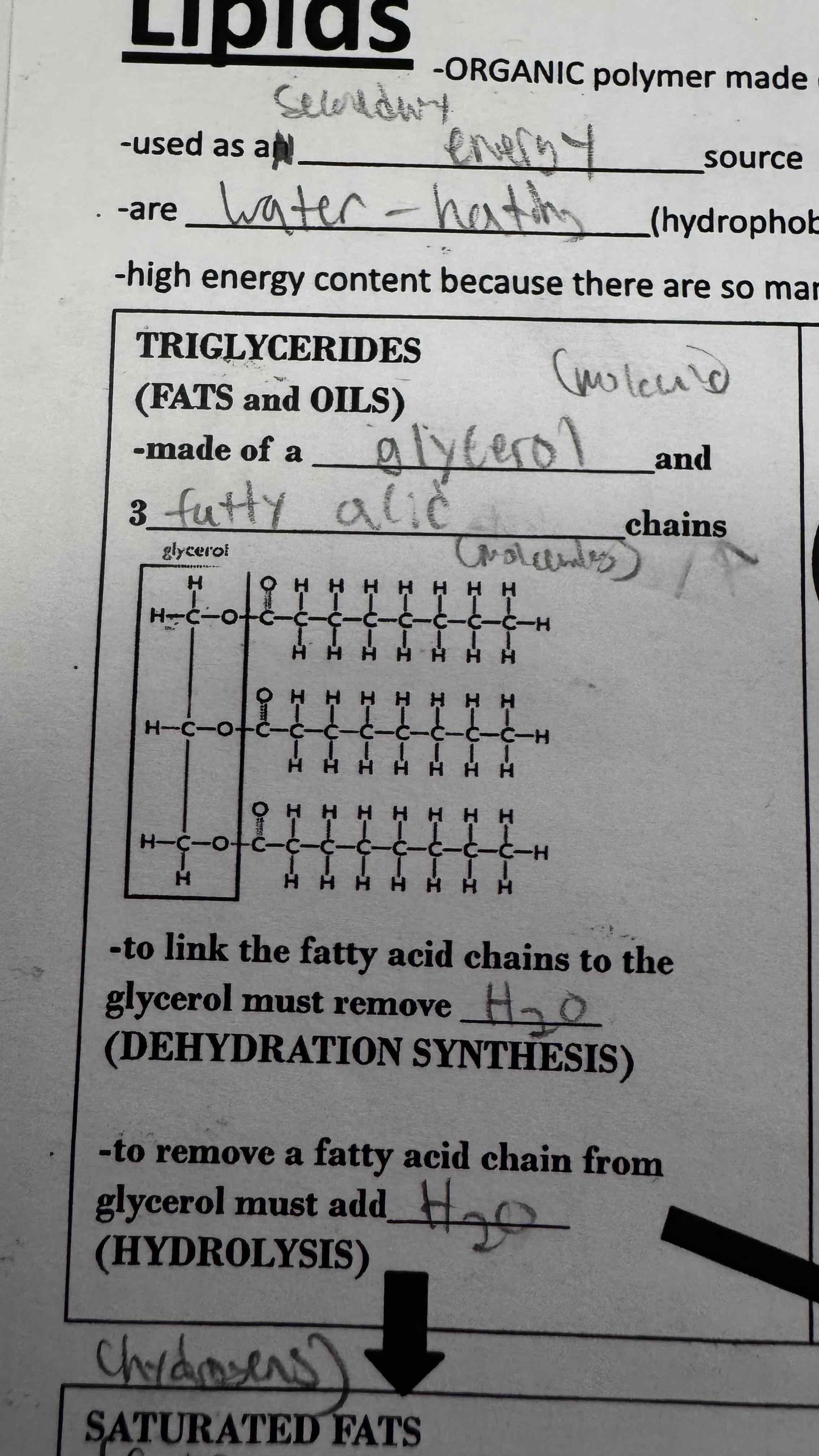
What type of lipid is this?
Triglyceride
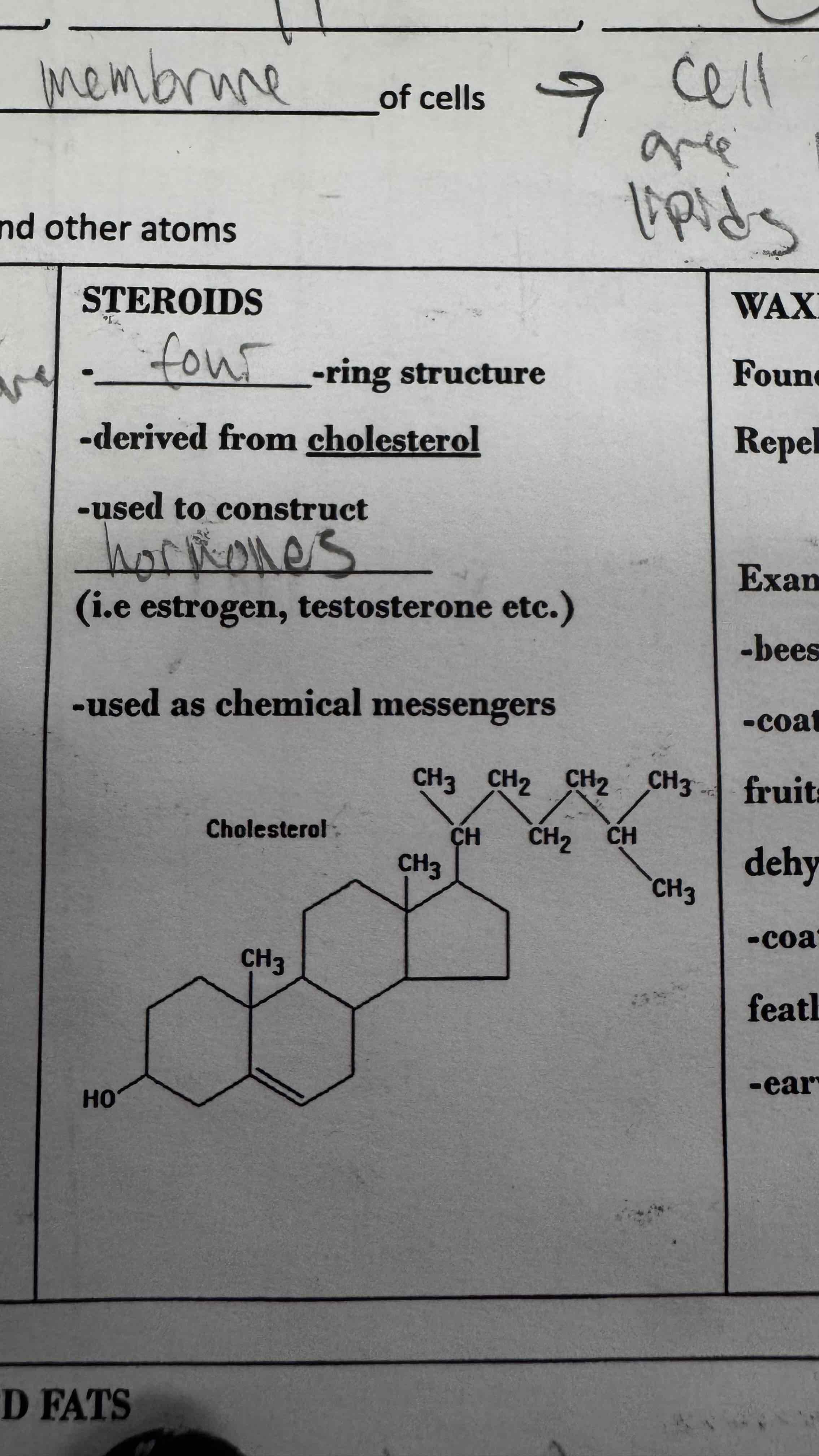
What type of lipid is this?
Steroid
Polypeptide

Mono saccharide

Poly saccharide
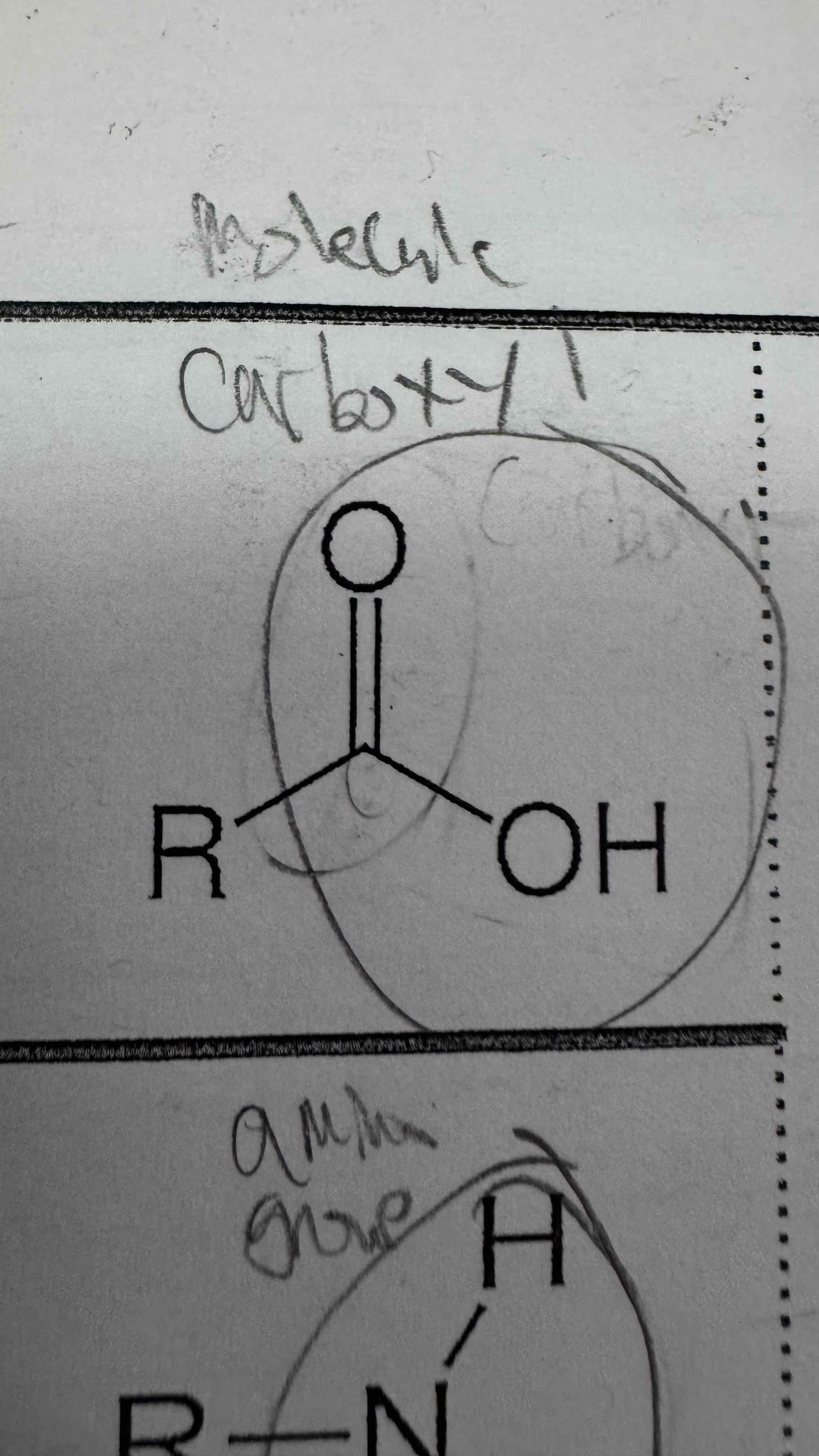
Carboxyl
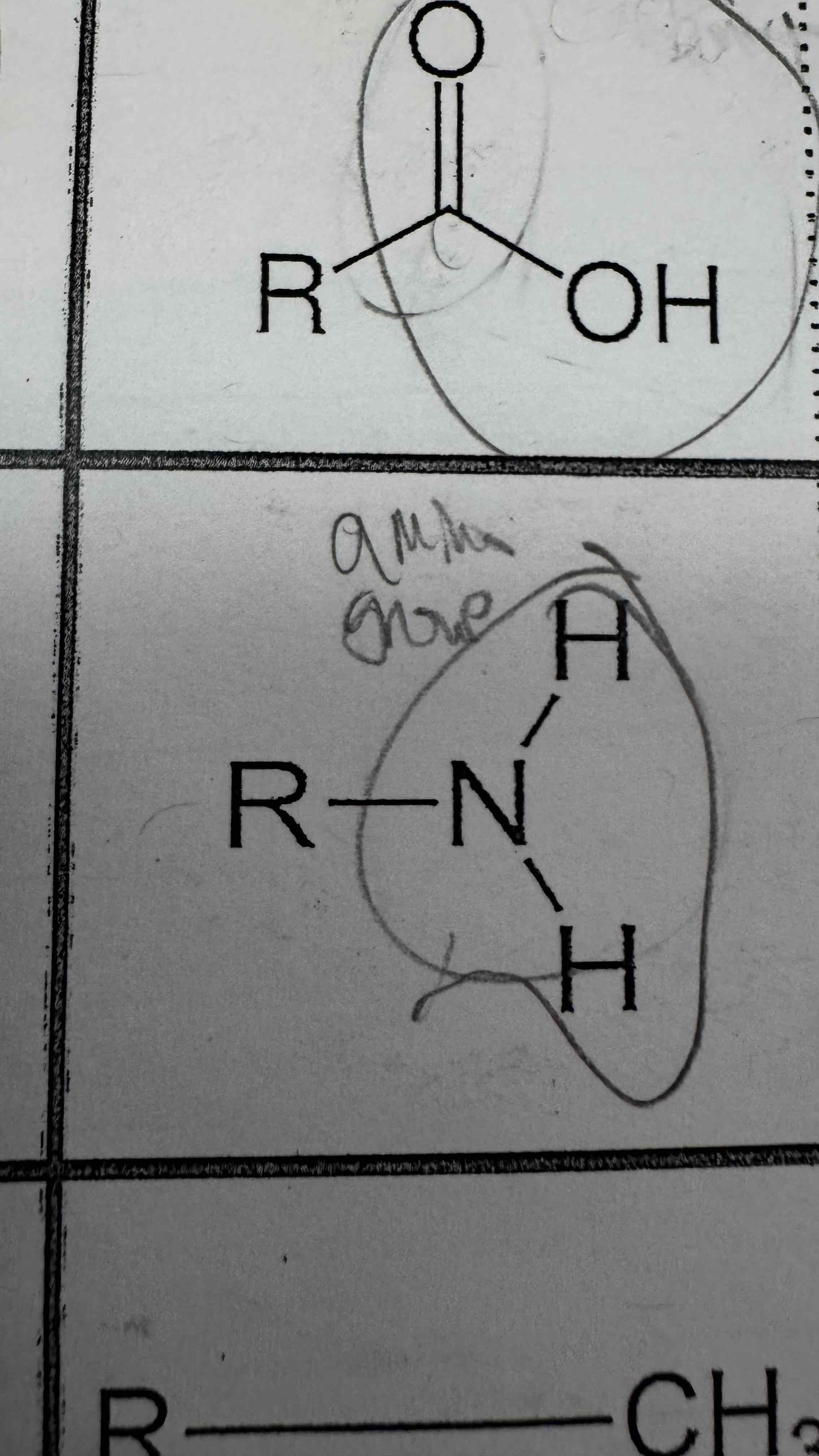
Amino group
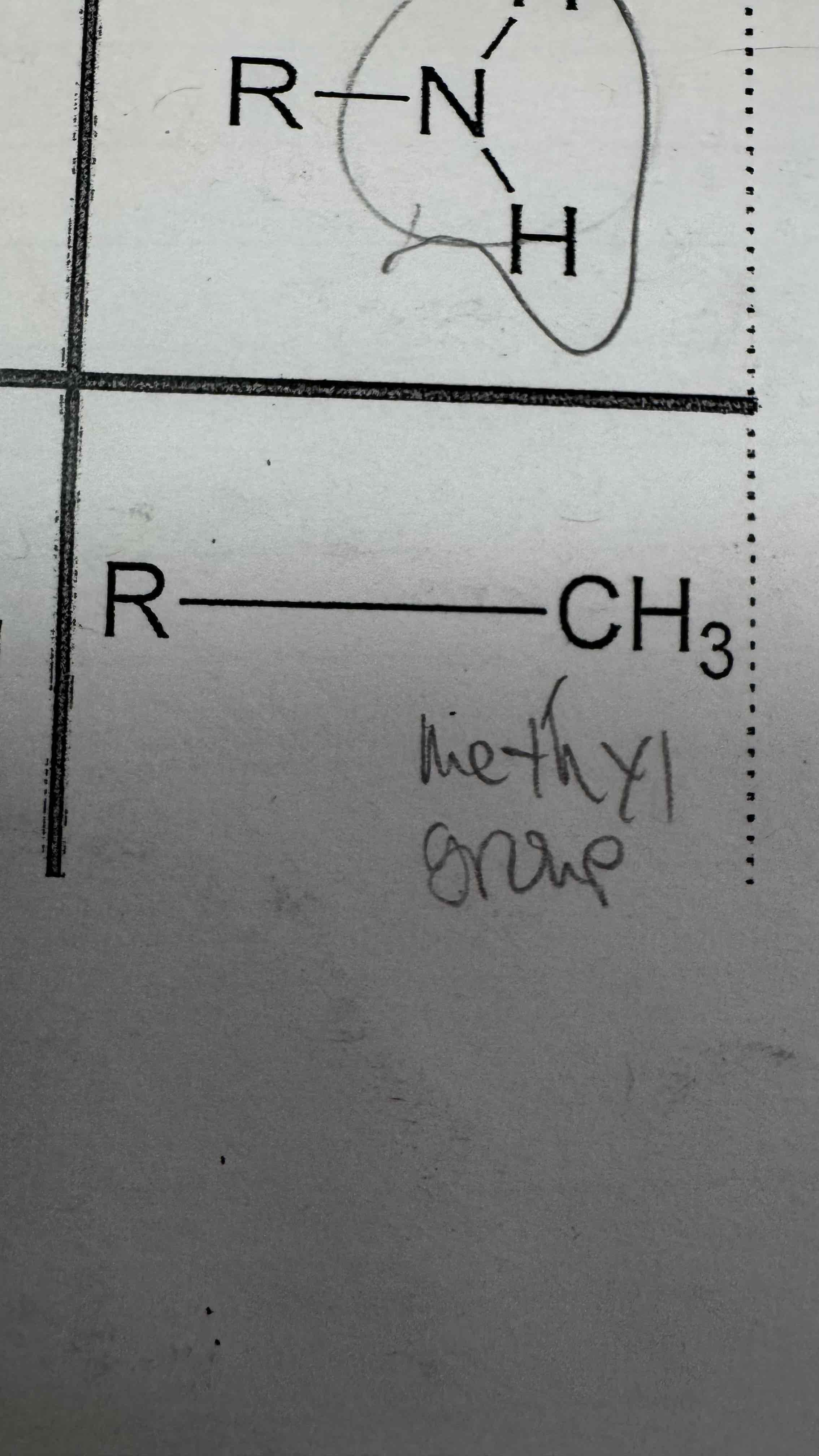
Methyl group
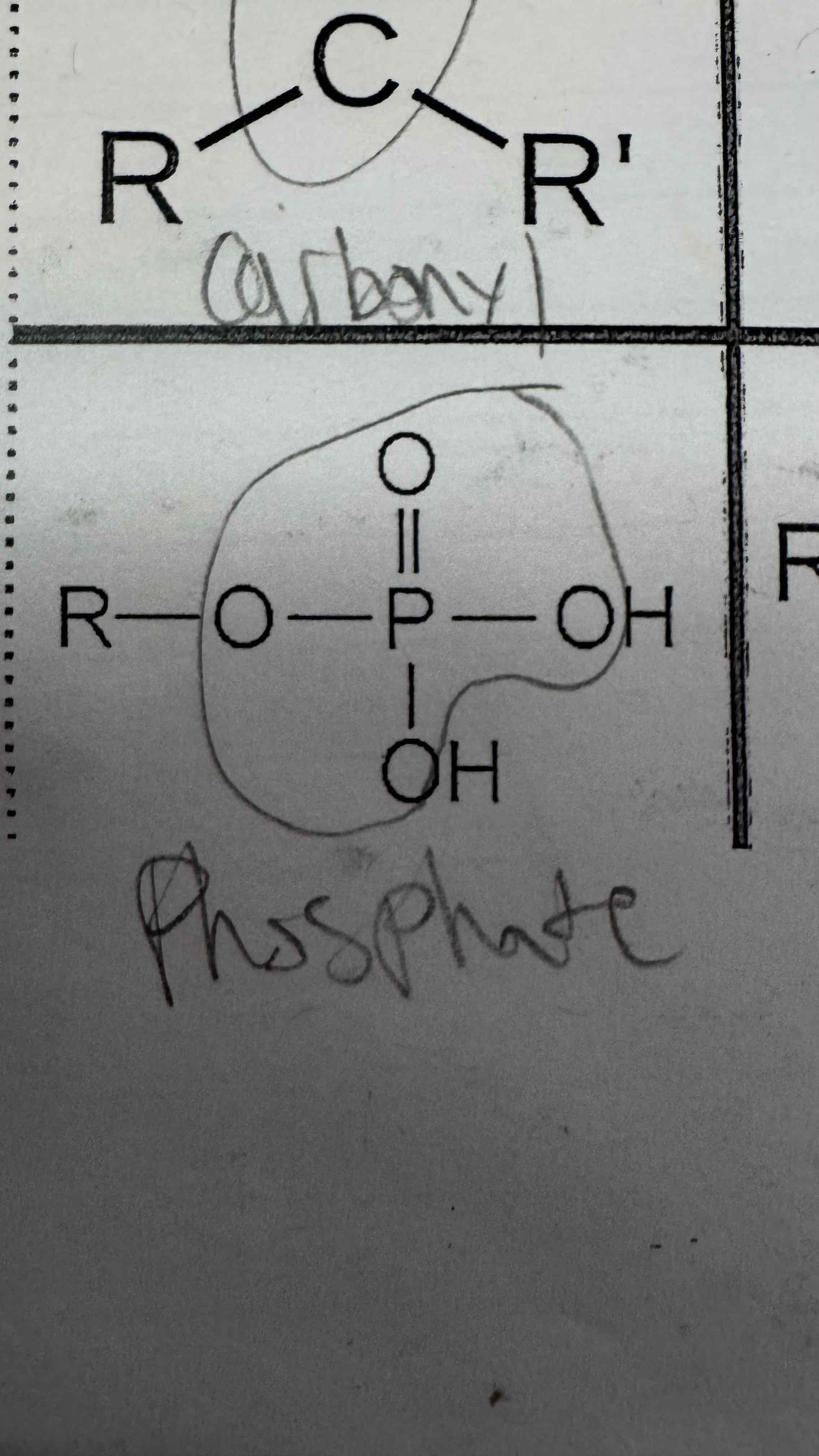
Phosphate
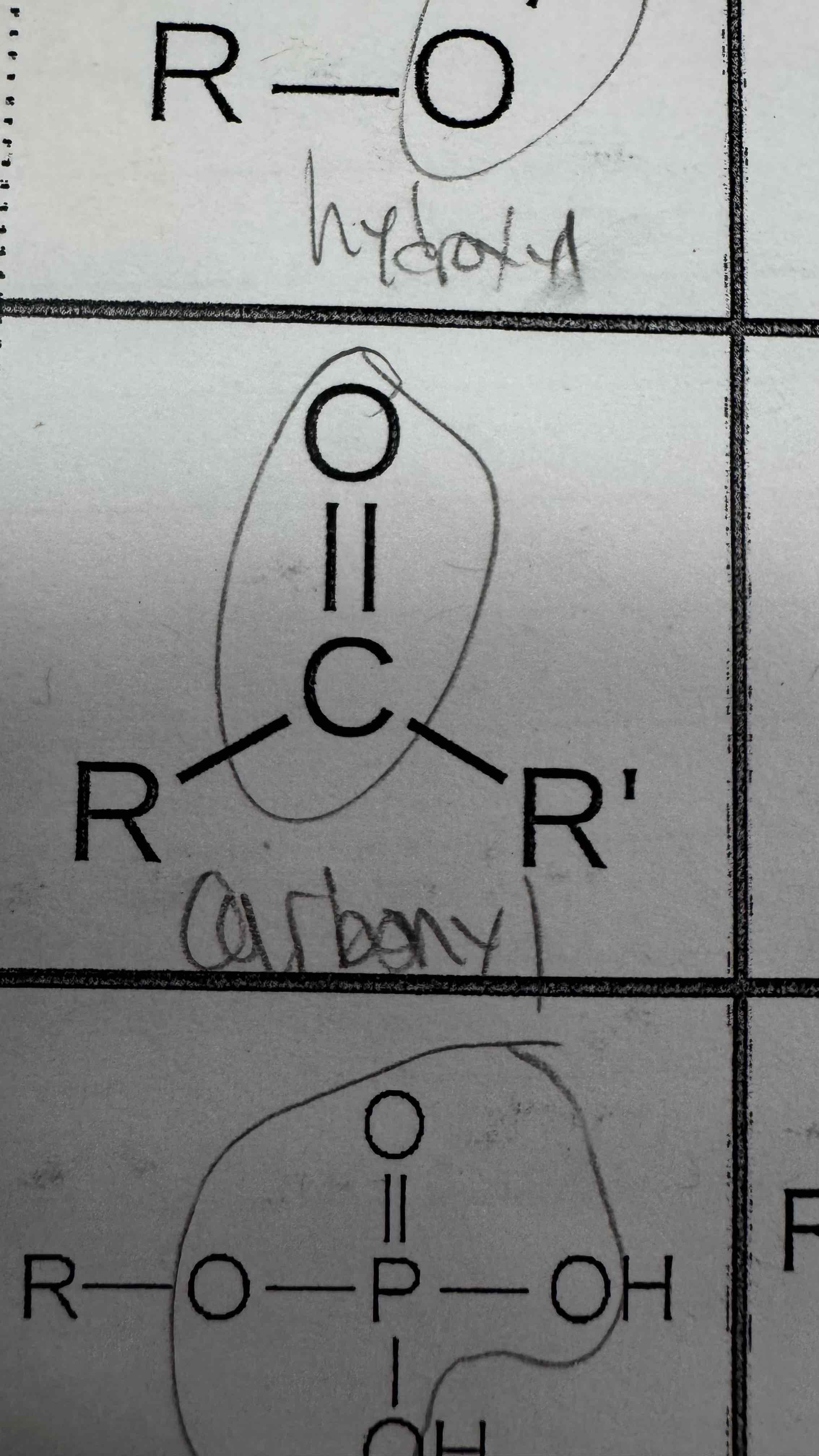
Carbonyl
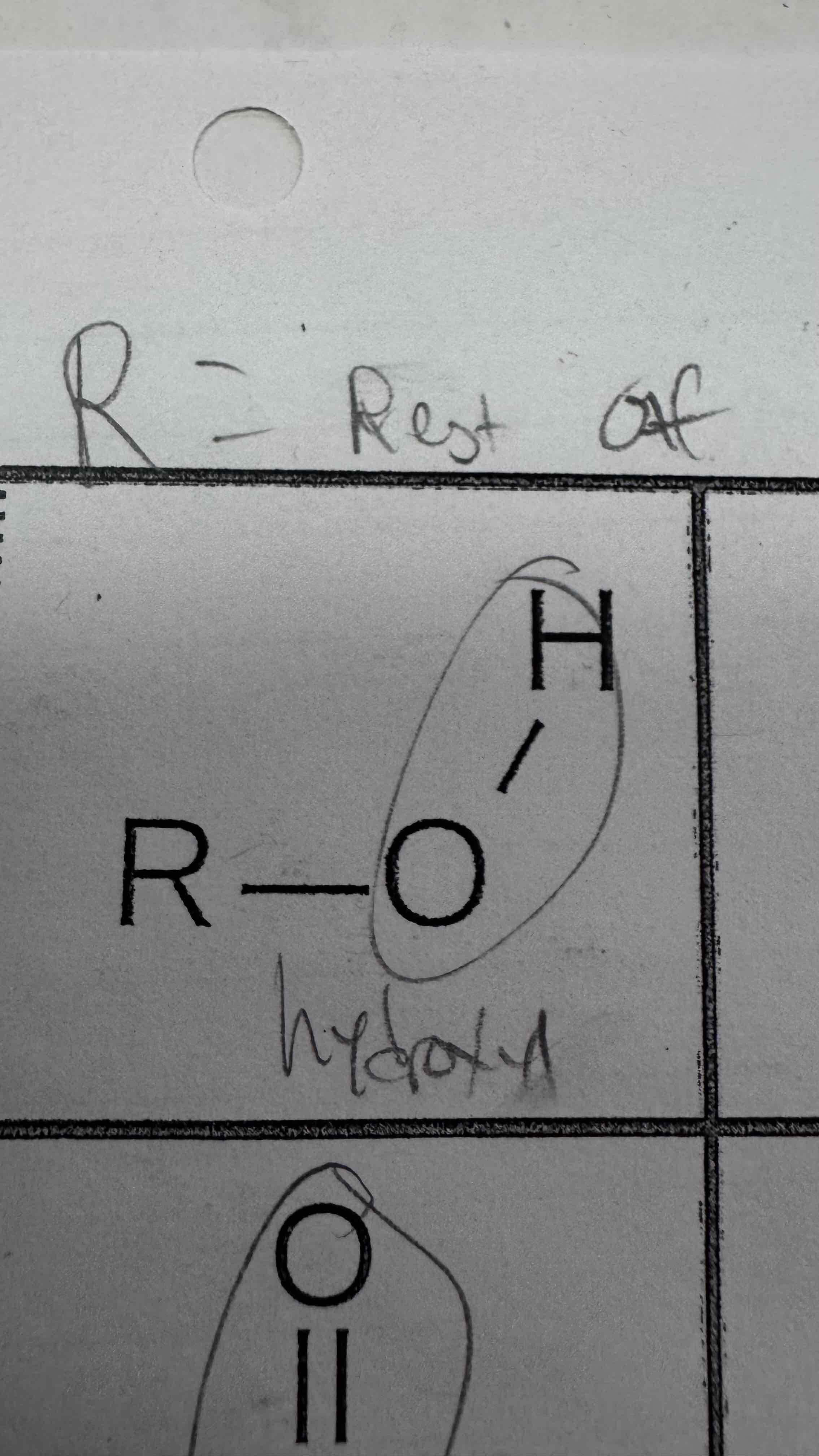
Hydroxyl