SOM1 - Colloidal dispersions
1/38
Earn XP
Description and Tags
Lecture 38+39
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
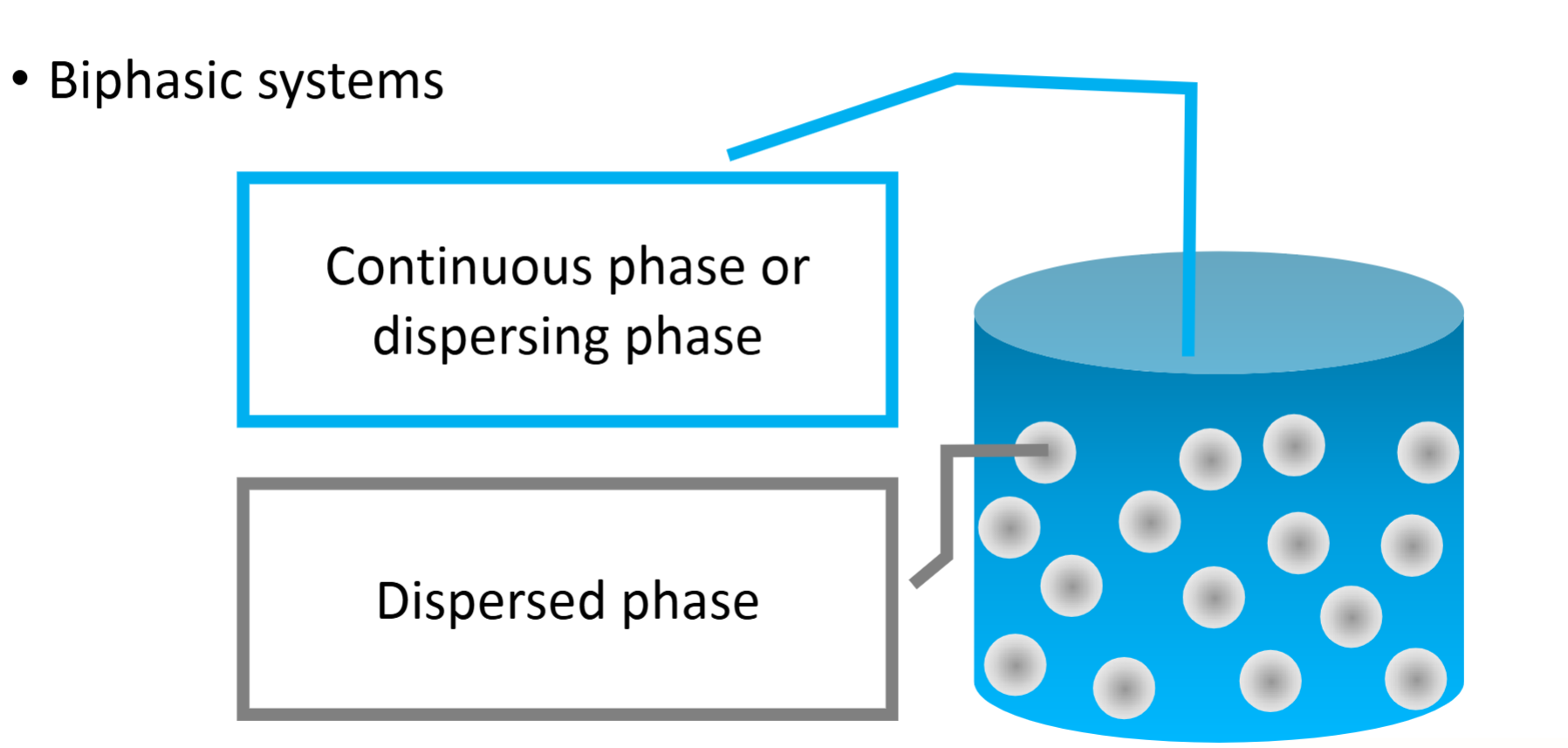
Disperse systems
What are the 3 size catergories of dispersions and their ranges
Coarse dispersions
10-50 mcm
Fine dispersions
1mcm-10mcm
Colloidal dispersions
1-1000nm or 1-500nm for some resources (in the nanometre range)
Molecular dispersions
<1nm
Basically a solution, as the dispersed phase is so fine
Difference between gels and sols
Sols:
Low viscosity
Gels:
High Viscosity
Dispersed and continuous phase particles intertwined
Name the 2 Classifications of dispersions by viscosity
Sols - LOW viscosity
Gels - HIGH viscosity and intertwined particles
can be described as a hydrogel/oleogel/alcogel depending on continuous phase
What would a system where the two phases have low affinity and a continuous phase of water be called
A dispersed system with a water continuous phase and a low affinity between phases would be a hydrophobic dispersion
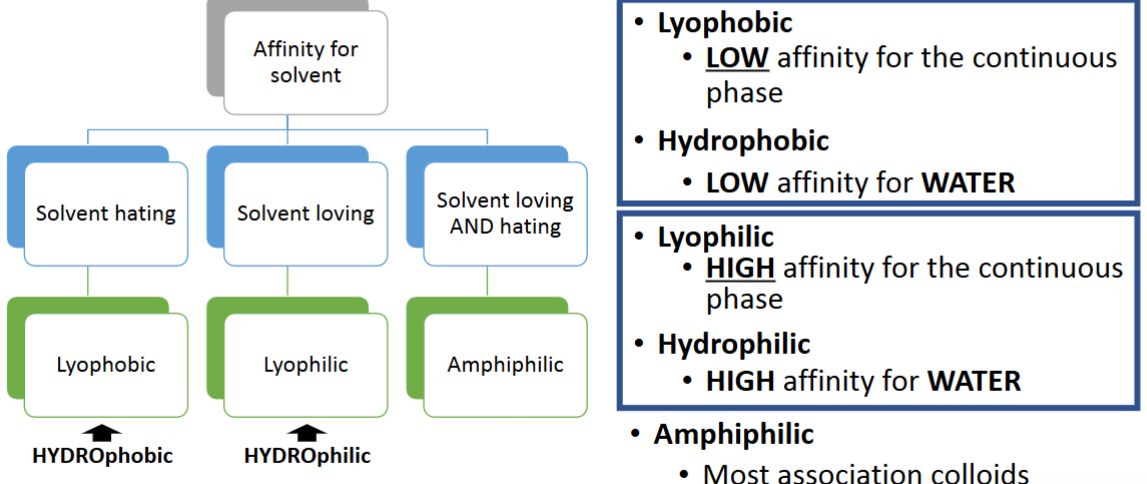
Lyophobic colloids characteristics
Possess low affinity for the continuous phase
They form thermodynamically unstable dispersions
Energy equation for lyophobic colloids
Gamma = surface tension
A = area
need to remember
Lyophobic colloid solvent order
Very ordered solvent molecules, because they don’t want to touch the central molecule
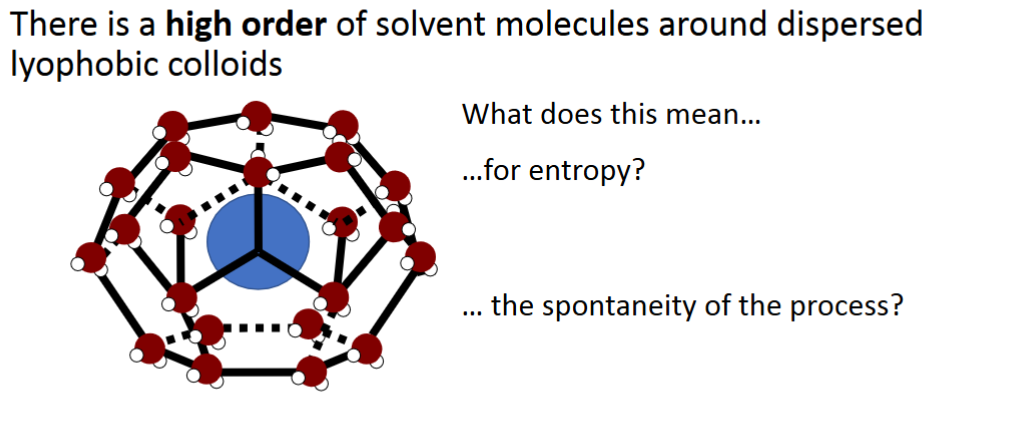
How does forming a lyophobic colloid affect the entropy in the system
Forming a lyophobic colloid causes a highly ordered structure of solvent around the colloid, the increased order decreases entropy and therefore also decreases spontaneity
Name 2 methods of preparing a lyophobic colloid
Breakdown of large particles via mechanical force
Controlled aggregation
Chemical reactions
Oxidation, reduction, hydrolysis etc.
Solvent changes
Colloids that are in a good solvent being transferred to a bad solvent
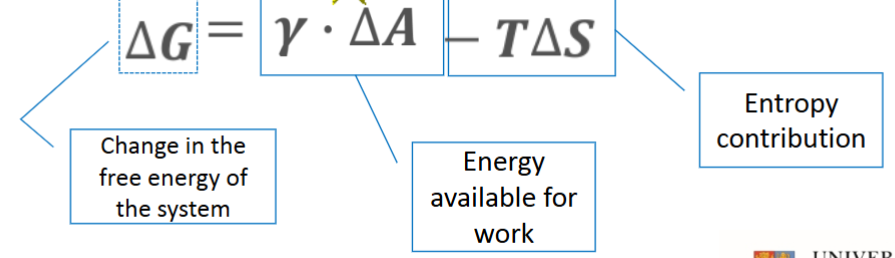
Using the Gibbs Free Energy equation, explain why lyophilic colloids form spontaneously
ΔG = γ ΔA - T ΔS
If there is high affinity between the continuous and dispersed phase, then the (γ ΔA) section of the equation is nearly negligible, as the change in area is minor. This means the (T ΔS) section is larger, and so gives a negative Gibbs free energy, and means the reaction needs no additional energy to occur (spontaneous)
Association colloids
Micelles behave like lyophilic colloids
Spontaneous formation above a minimal concentration
Thermodynamic stability
Micelles are typically spherical and made of a number of surfactant molecules (aggregation number)
Not all amphiphiles form micelles
Different types of shape
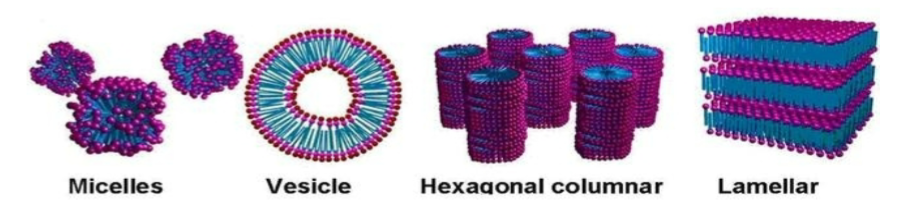
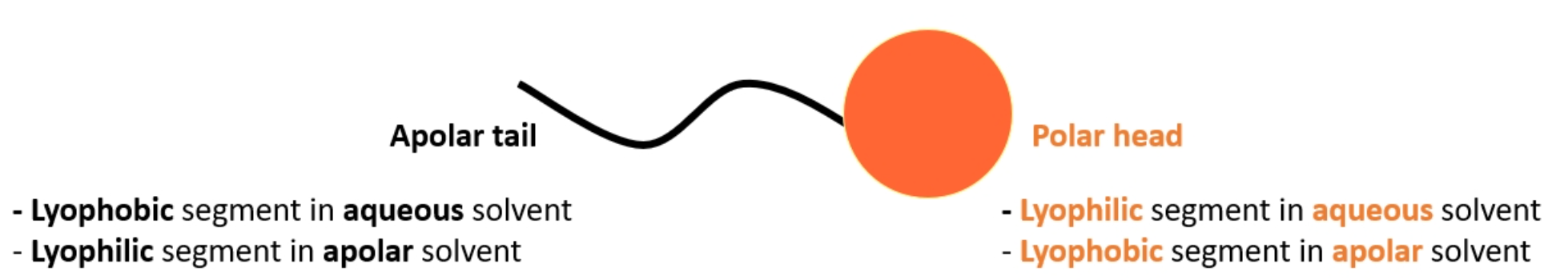
What type of colloid do surfactants behave like and why
lyophilic, as there will always be a part of the molecule that likes the continuous phase, it will just flip depending on medium (polar head inside and nonpolar tails outside in a oil, and vice-versa in water)
They also have spontaneous formation (when above a minimal concentration (CMC))
And thermodynamic stability (up to a point)
How do surfactants reduce surface tension?
Their amphiphilic structure allow them to act as a bridge between the molecules and disrupt the stronger cohesive forces between the bulk molecules. This brings the two phases closer together and decreases surface tension
How can you tell when a dispersion is saturated with surfactants (reached its critical micellar concentration)?
When surface tension stops dropping, as that means there is no more surface for the surfactant to attach to, and so stops dropping surface tension
What do the surfactants do when the dispersion is oversaturated with surfactants
They form micelles of just surfactant to protect the phobic part of the surfactant molecules
What effect does micelle formation have on entropy, and is it energetically favourable
Increases entropy, as lone surfactant molecules require a large amount of water molecules around them to form the ordered structure around it, but when surfactants combine to form a micelle, less water molecules are needed
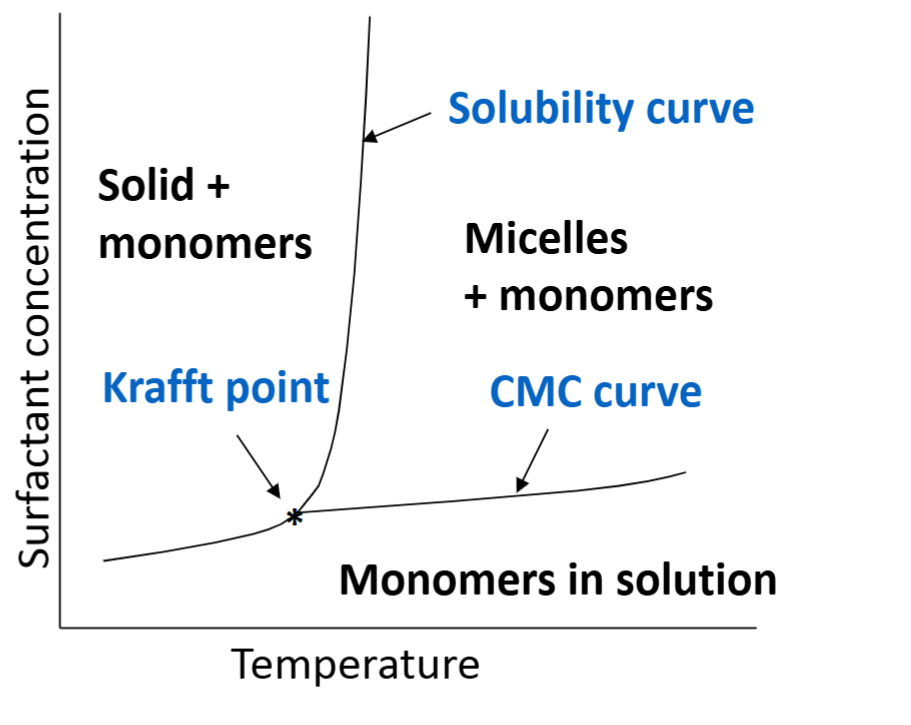
What is the Krafft point?
Temperature above which the solubility of a surfactant increases sharply (critical micelle temperature)
At temperatures below Krafft point, surfactants just precipitate instead of forming micelles.
Basically the minimum temperature for any meaningful amount of micelles to form
what is the cloud point
Temperature above which the solubility of a surfactant decreases sharply (basically opposite of Krafft point)
Cloudy appearance is due to polar head being dehydrated, and precipitating
Reversible, can be fixed by cooling
What causes shape variety of dispersed phase components in colloids
Affinity with solvent
In a good solvent, what are the properties of a colloid in regards to:
Lyophilic/lyophobic
will it want more or less interaction with dispersing phase
Will it form a scrunched or extended shape
Contribution of surface tension to overall free energy
Dispersion spontaneity
Lyophilic
More interactions
Extended - wants to maximise contact with dispersing phase
Surface tension will contribute almost nothing
Spontaneous dispersion, no additional energy required
What is dialysis
Separation of colloidal particles from small molecules/ions
How is dialysis performed
Semi permeable dialysis membrane allows ions and molecules to diffuse, but not colloids because they’re too big.
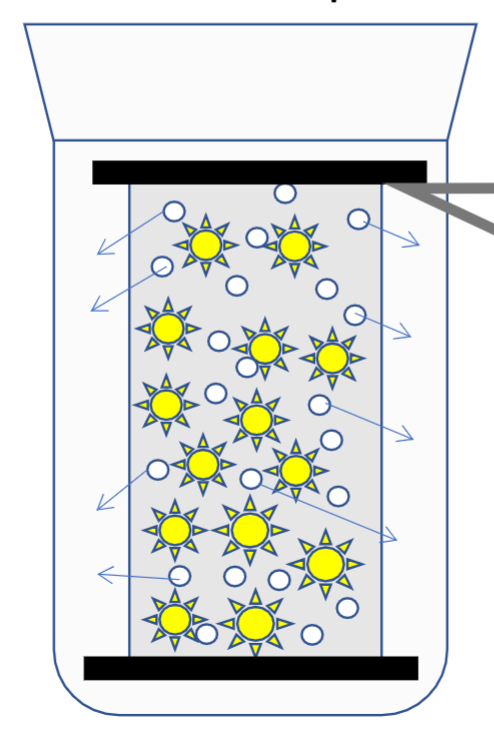
Kinetic properties of colloids to think about
• Brownian motion
• Diffusion
• Sedimentation
• Viscosity
• Osmotic pressure
Colloids contribute to total osmotic pressure
• Donnan membrane effect
Impact of charged colloids on diffusion of small ions across a membrane
Brownian motion
Random movement under thermal agitation
only affects particles up to 5 micrometers
Why is brownian motion relevant in regards to sedimentation
It is what stops colloidal dispersions from spontaneously sedimenting, as it provides an upwards force.
Sedimentation
Downward motion under gravitational forces. Due to Brownian motion, will not spontaneously occur under normal gravity, may need centrifugation.
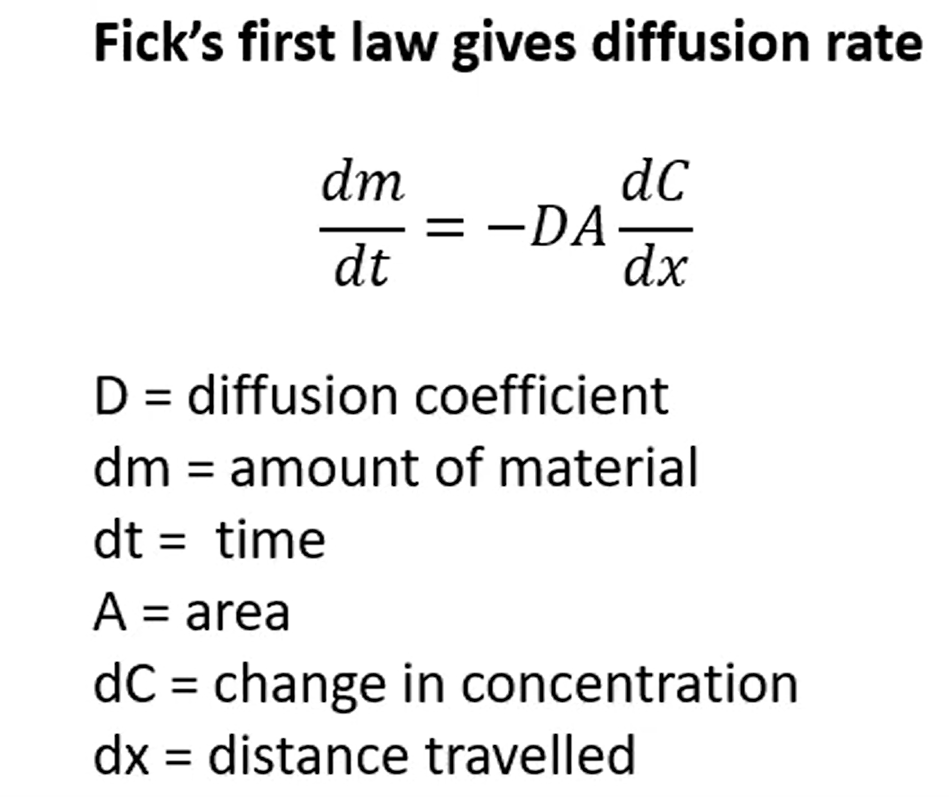
Fick’s first law + what is it used for
Diffusion rate
Viscosity
Resistance to flow under an applied force
Can be used to determine molecular weight of lyophilic colloids
Changes with:
Solvation state (basically how much continuous and dispersed phase interacts, higher solvation state = more interactions)
Increases with degree of solvation
Shape
Spherical vs elongated
Concentration
Increases with concentration
Molecular weight
Increases with the molecular weight of the colloid
Why does a colloidal dispersion look blue and cloudy under a light
Faraday-tindall effect causes the dispersion to scatter light, causing a cloudy look, and blue wavelengths are shortest, so they scatter more
Electrical properties of colloids
A partticle may become charged through:
Being naturally charged
Ion adsorption
Adsorption of oppositely-charged ions from dispersed phase
Ion dissolution
Excess ions in solution
Ionisation of surface groups
Ionisation of surface groups
Permanent/pH-dependent charge (e.g. acids & bases)
Affects distribution of other ions
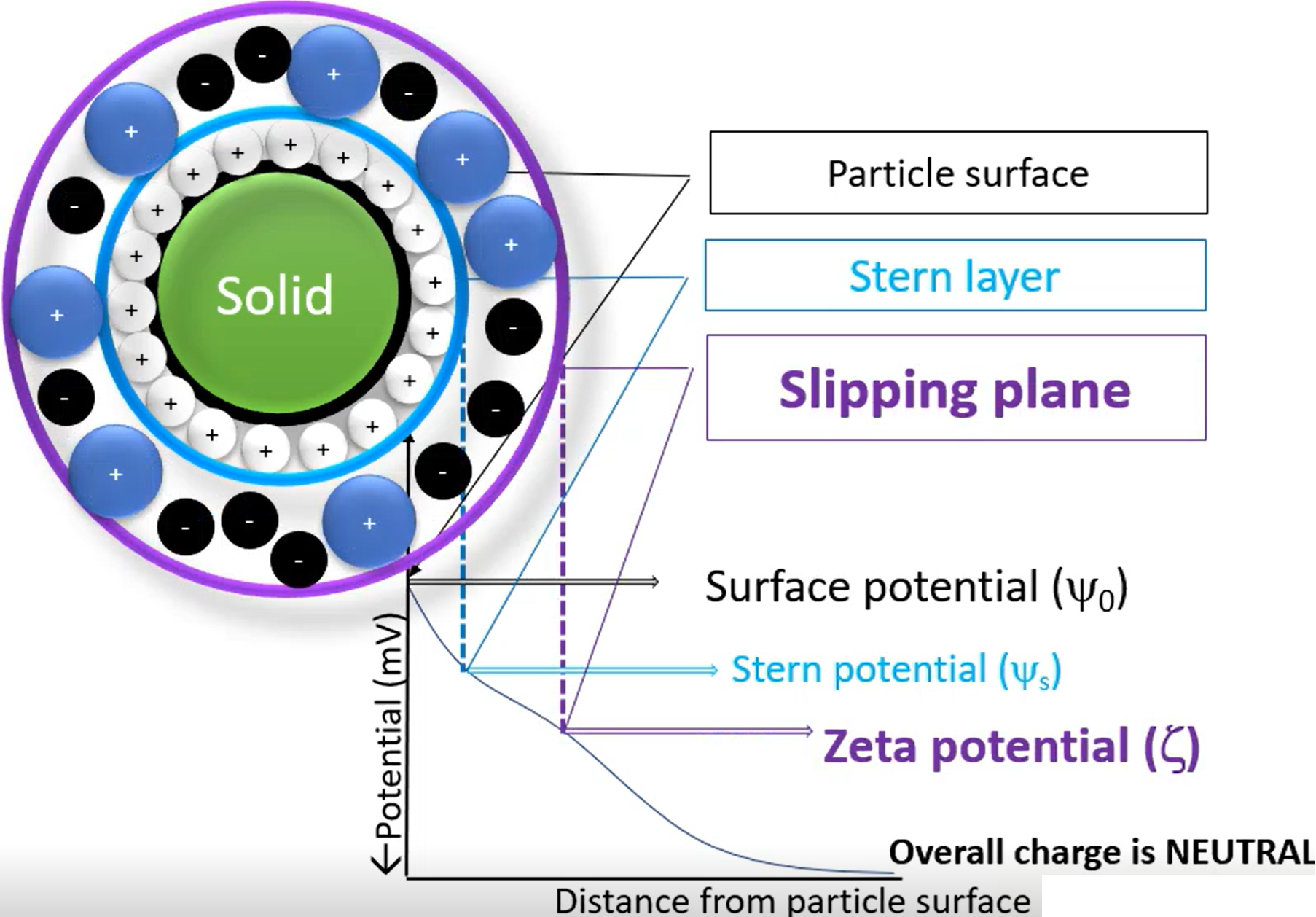
describe particle surface, stern layer and slipping plane
Particle surface - Just the surface of the dispersed phase in the suspension, no additives
Stern layer - If the particle surface is negatively charged (for example), then it will attract positively charged cations, which forms a layer around the surface (the ions are not the dispersing phase, just other random ions in the solution)
Slipping plane - Other ions may be pushed in/out by the stern layer, causing one final layer to form around it. The potential around the slipping plane is the Zeta potential, and is essentially the overall surface charge.
Stern and slipping plane both move with the particle
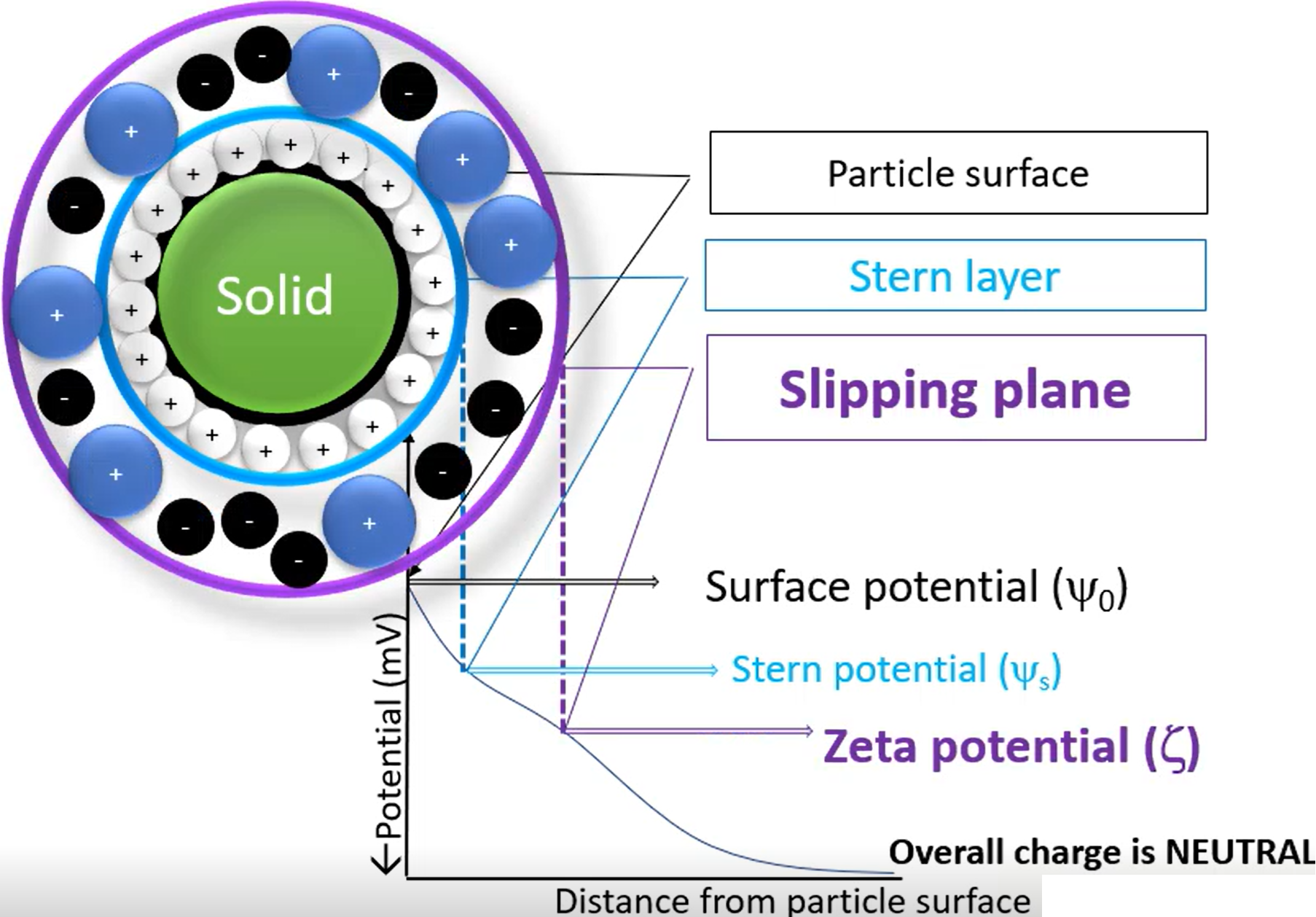
Zeta potential
Effective particle charge
contribution of ions in solution
Impacts on stability, moreso on hydrophobic colloids
Why do we measure zeta potential
Indicates stability
3 Major forces on stability of colloid
Repulsive
Electrostatic - highly charged particles will repel eachother
Attractive
Steric
Linked to solvation
shell of solvent around dispersed phase means it is more difficult for particles to aggregate
Stabilisation of suspensions
Means large, solid, poorly solvated particles can be covered in lyophilic colloids to form a stable suspension
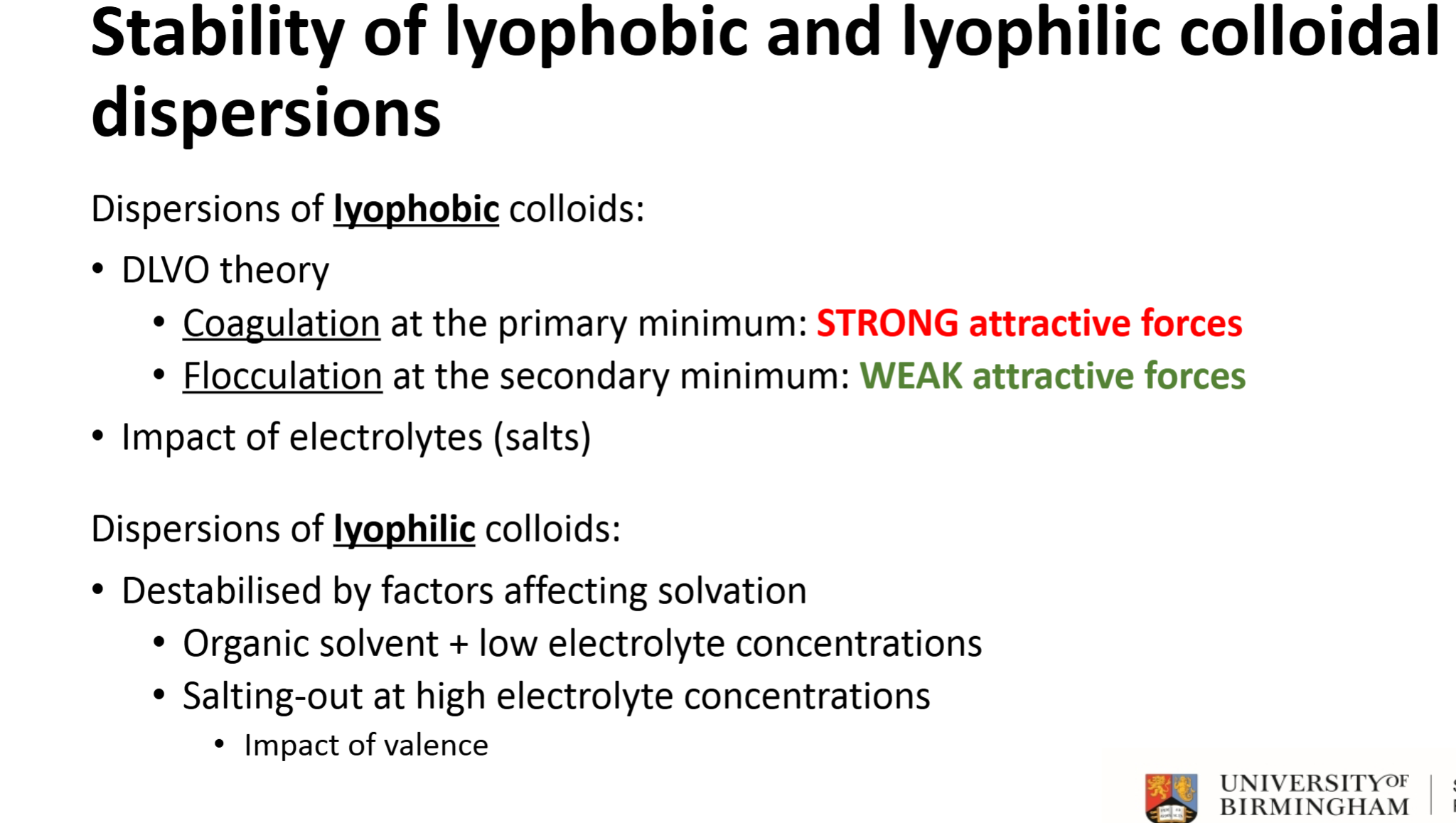
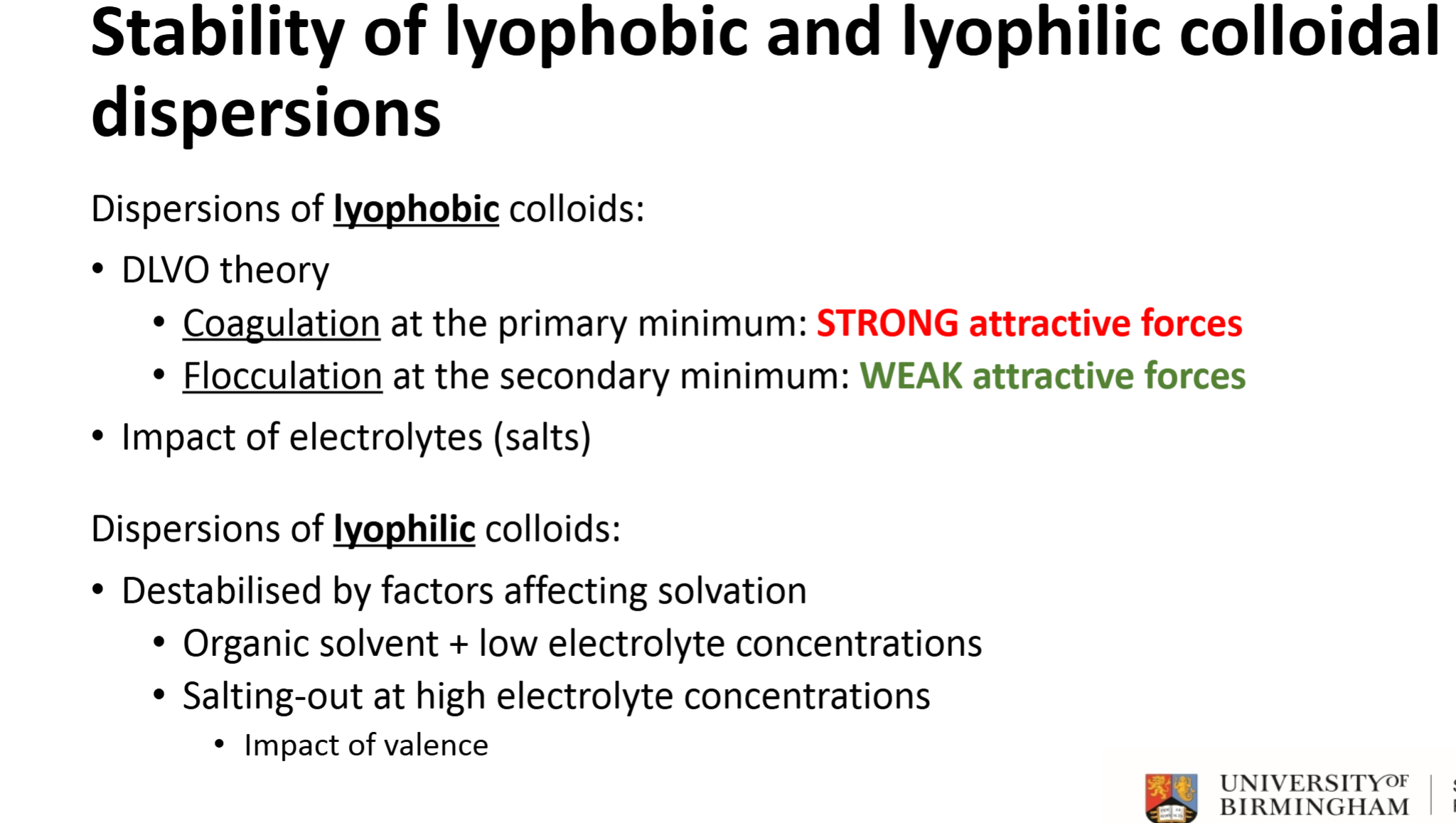
What does a weak attractive energy signify on the DVLO chart? (secondary minimum)
Flocculation - can easily redisperse particles by shaking
What does a strong attractive energy signify on the DVLO chart? (primary minimum)
Coagulation - shaking will not be strong enough to redisperse particles
How do you figure out total potential energy of colloid interaction
DLVO theory, compare attractive Van der Waals forces to repulsive electrostatic energy,
Good indicator as to whether the dispersion will stay aggregate or split
Stability of lyophobic and lyophilic colloidal
dispersions