P3 - Particle model of matter
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
How to measure the density of a regular shaped object?
Measure the mass using a top pan balance
Measure the length, width and height using a ruler and find the product to get volume
Do the equation Mass (kg) ÷ volume (m³) = density (kg/m³)
What is density
Measure of how much mass there is in a given space
What does a dense material have more of than a less dense material?
More particles in the same volume
When a substance changes state, is it a physical change or a chemical change?
Physical change
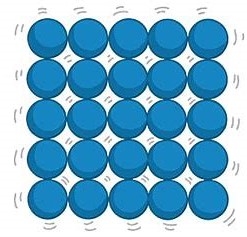
What do particles do in a solid?
Particles have strong forces of attraction; they are held together very closely in a fixed, regular arrangement. The particles do not have much energy and can only vibrate.
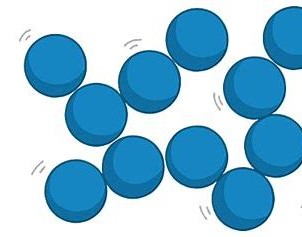
What do particles do in a liquid?
Particles have weaker forces of attraction; they are close together, but can move past each other. They form irregular arrangements and have more energy that particles in a solid
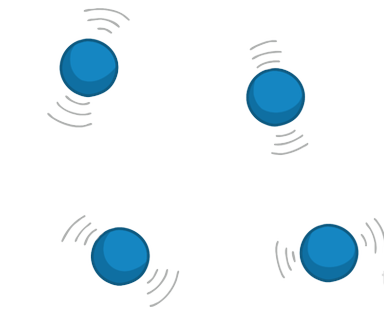
What do particles do in a gas?
Particles have almost no forces of attraction between the particles. They have the most energy and are free to move in random directions
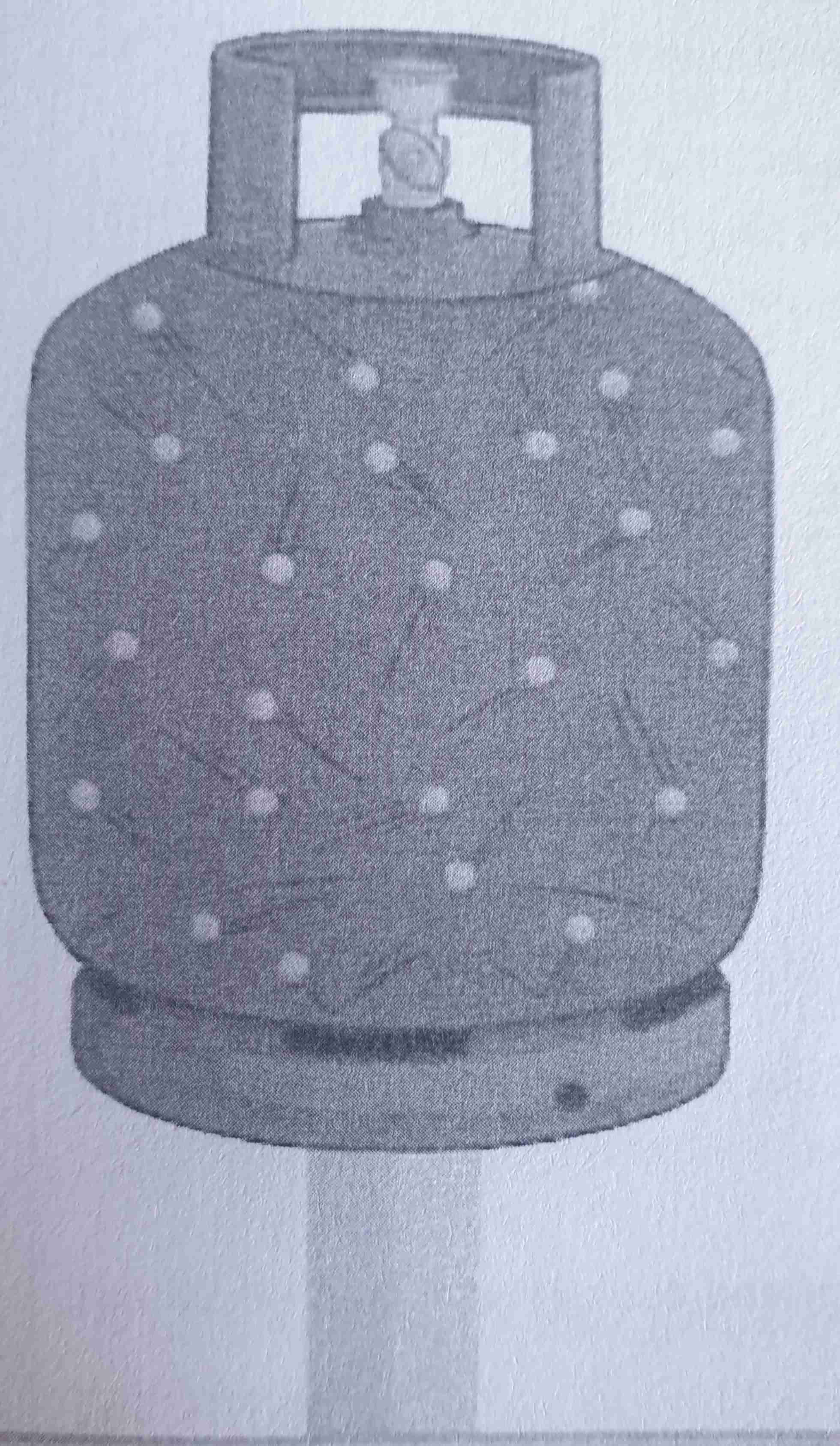
If gas is trapped in a container and heat is applied, what will happen?
The kinetic energy in particles increases, causing them to collide with the sides of the container more often. This increases the pressure of the container