DF8: Burning Fuels
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
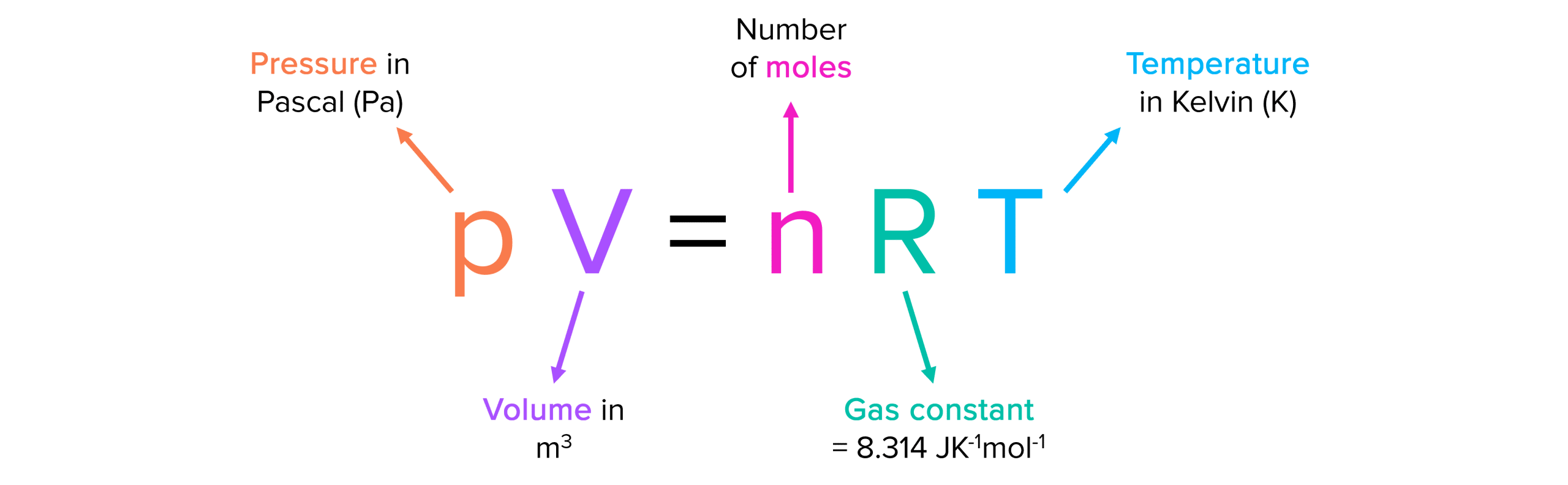
What is the ideal gas equation?
pV = nRT
2
New cards
How do you calculate the volume of gas produced in a reaction?
Use the balanced chemical equation to find the moles of gas produced.
Multiply the moles by the molar gas volume at RTP (24 dm³ mol⁻¹)
3
New cards
How do you calculate the mass of gas from its volume?
Calculate the moles using moles = volume ÷ molar gas volume.
Use mass = moles × molar mass
4
New cards
What is the molar gas volume at standard temperature and pressure?
At STP