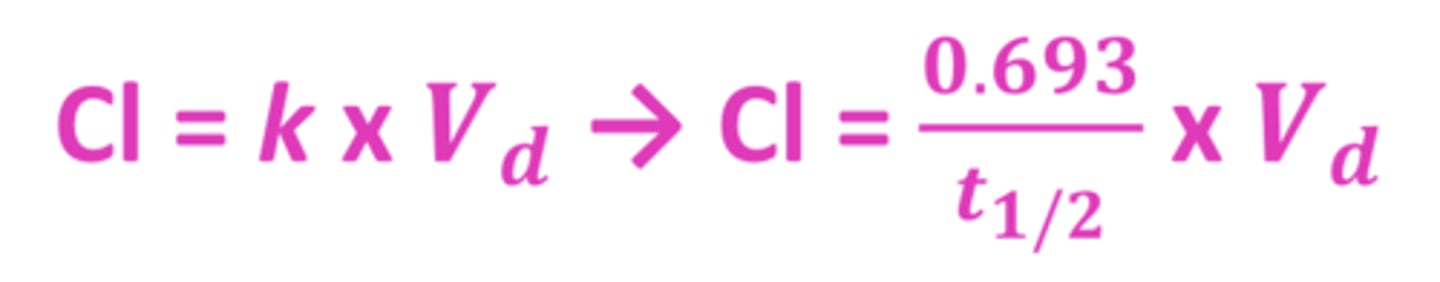(FORMULAS) Rate constants: Zero and first order kinetics, Half-life, Volume of distribution, Clearance
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Last updated 3:34 PM on 5/19/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
1
New cards
First-Order Kinetics/elimination formuala
A = A0e^(-k)(t)
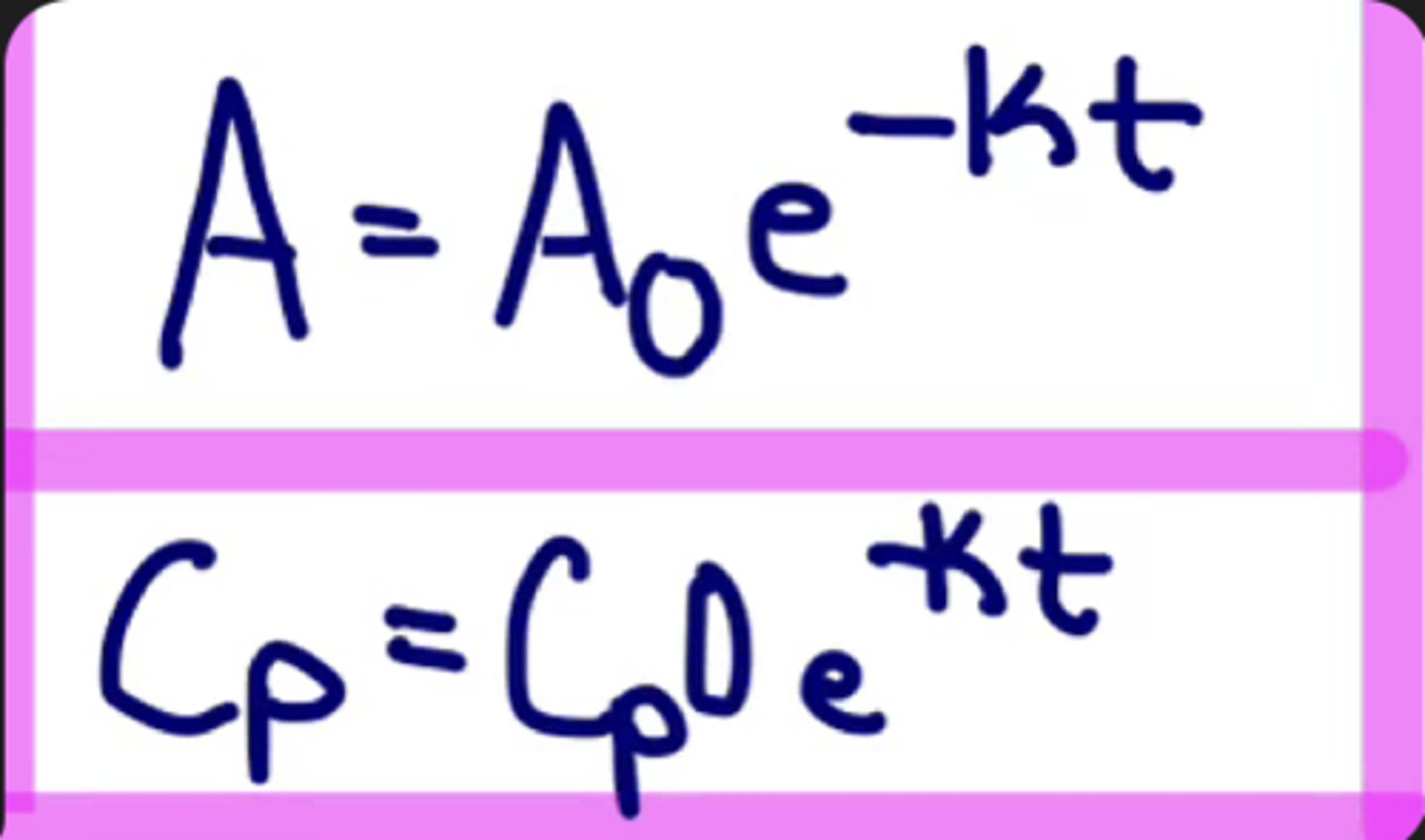
2
New cards
First Order Half-Time formula
(for first-order, half-life does NOT depend on [A] (concentration)
![<p>(for first-order, half-life does NOT depend on [A] (concentration)</p>](https://knowt-user-attachments.s3.amazonaws.com/871fcffc-c9e3-4ba9-87d8-7b872b7a0376.png)
3
New cards
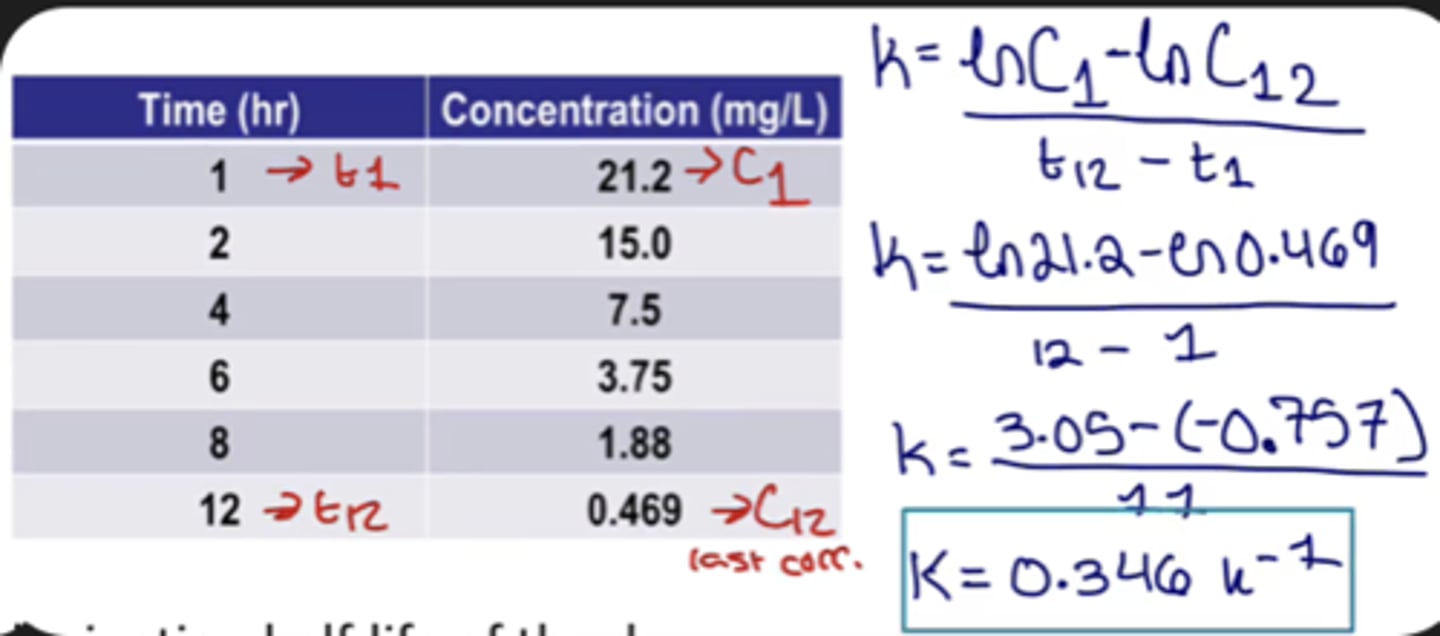
Formula for finding K from time and concentration (chart)
4
New cards
Formula for finding K from half-life
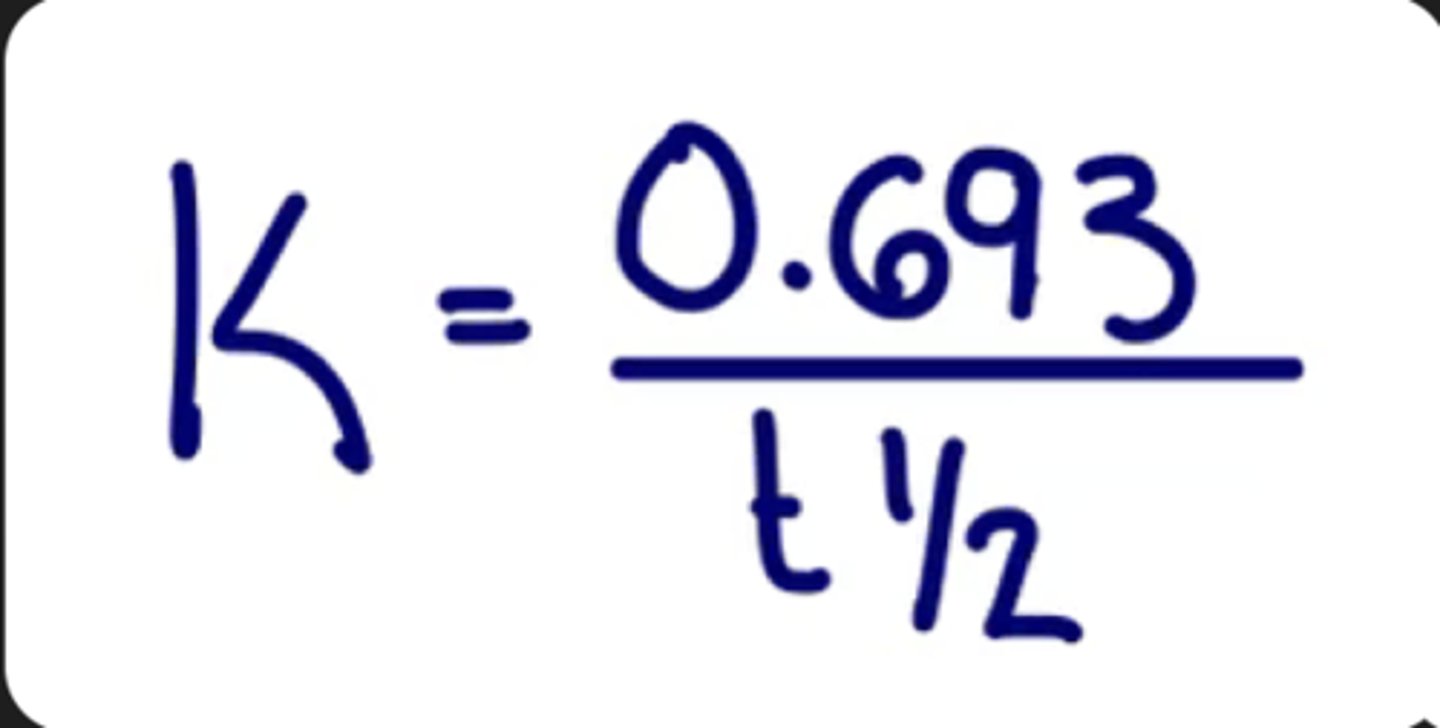
5
New cards
Zero-order Half-Time formula
dependent on [A] (concentration)
![<p>dependent on [A] (concentration)</p>](https://knowt-user-attachments.s3.amazonaws.com/cc56b1e8-44b5-45de-96dc-ecd21dc5655e.png)
6
New cards
First-Order Vs. Zero-Order reaction: Half times and chart
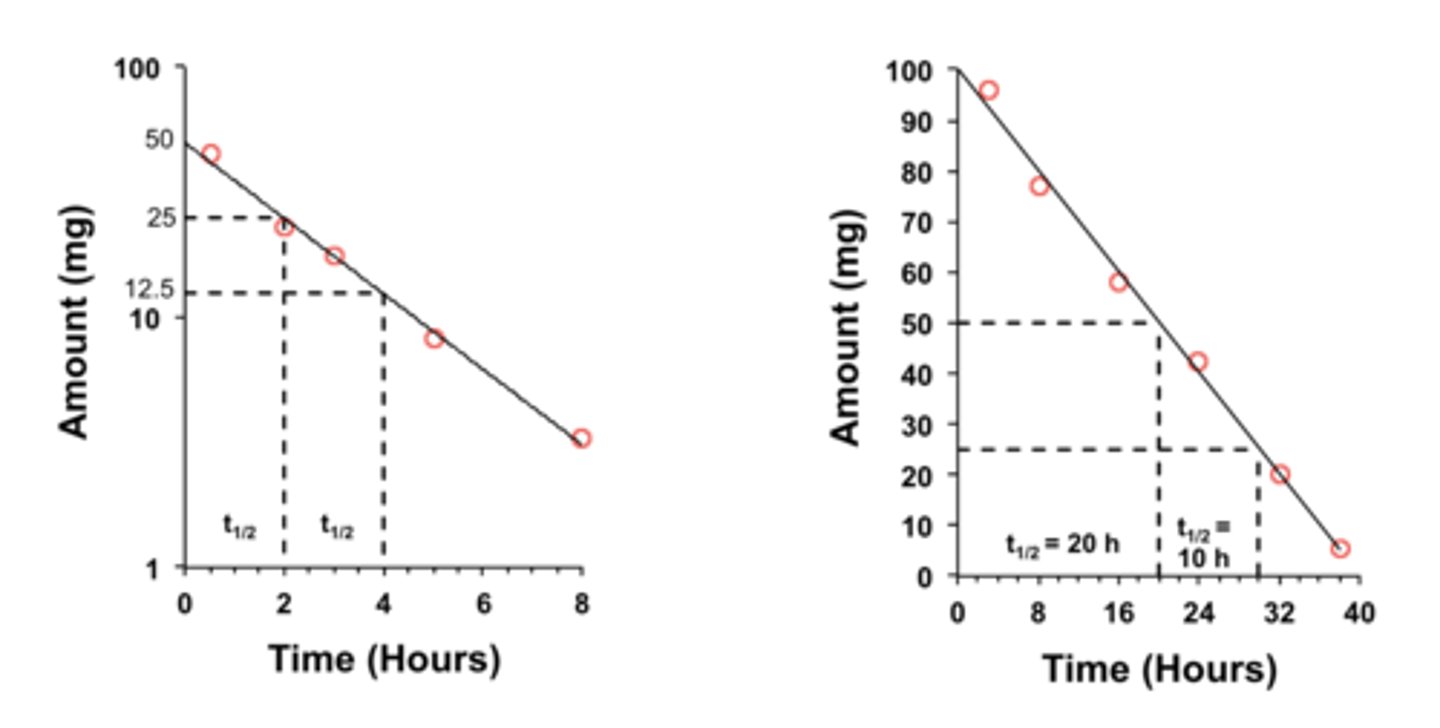
7
New cards
Volume of distribution formula
units of volume or volume/weight
(ml, L, or ml/kg, or L/kg)
- D0 = VD * Cp0
- Cp0 = DO/VD

8
New cards
Clearance formula
units in volume/time (L/hr, mL/min)
or volume/time/weight (L/hr/kg)