Biochem - 9.3 notes (book + lecture)
1/338
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
339 Terms
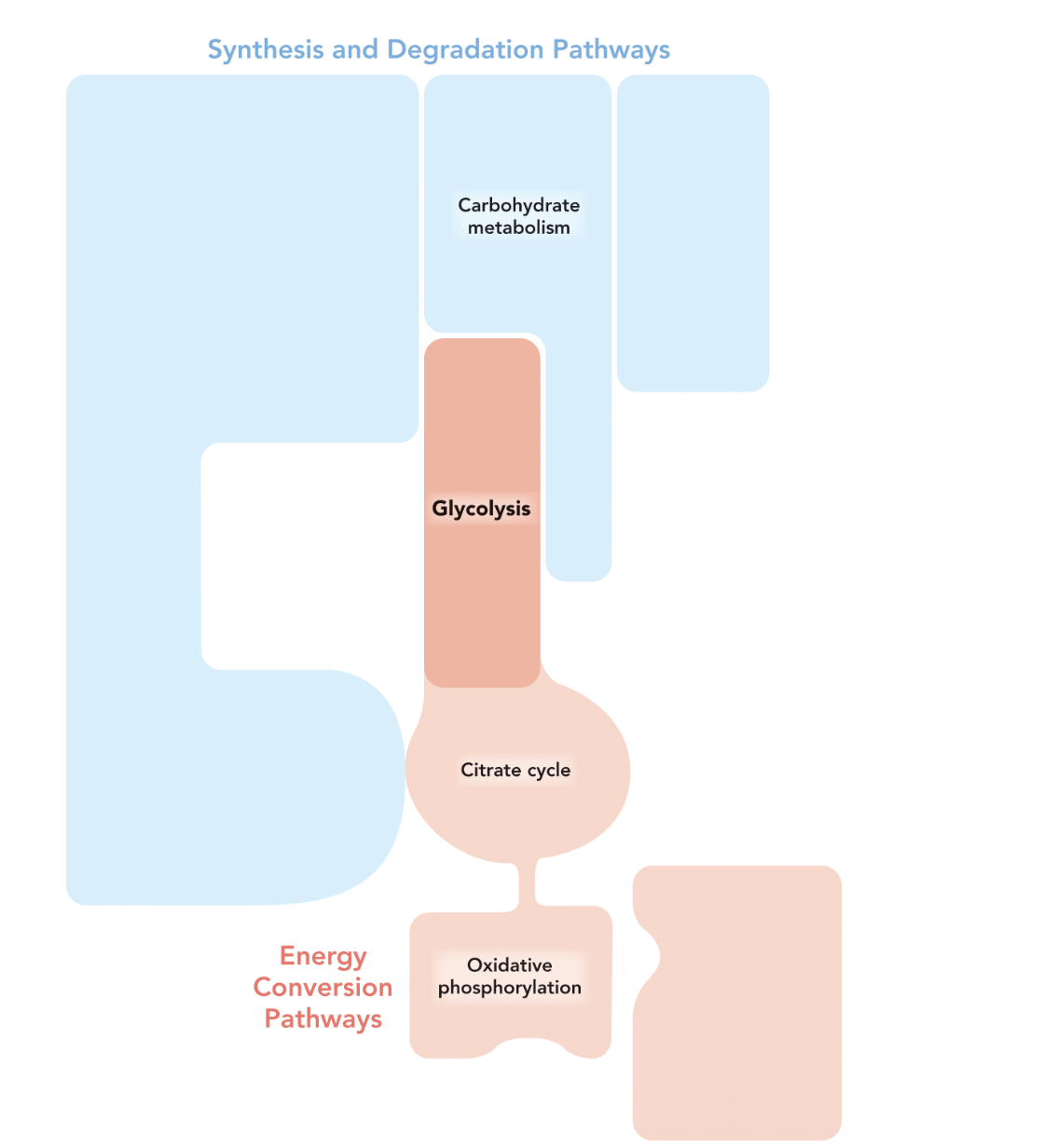
Q: What does Figure 9.15 show?
A: Figure 9.15 shows glycolysis at the top of a set of interconnected pathways that include the citrate cycle and oxidative phosphorylation. Together, these pathways completely oxidize glucose to CO₂ and H₂O.
Q: What is the overall oxidation reaction of glucose?
A:
Glucose (C₆H₁₂O₆) + 6 O₂ ⇌ 6 CO₂ + 6 H₂O
ΔG°′ = −2867 kJ/mol
ΔG = −2938 kJ/mol
Q: Why is glycolysis considered a core metabolic pathway?
A:
Glycolytic enzymes are highly conserved among all living organisms → ancient pathway.
Primary pathway for ATP generation under anaerobic conditions and in cells lacking mitochondria (e.g., erythrocytes).
Glycolytic metabolites are precursors for many interdependent pathways, including mitochondrial ATP synthesis.
Q: (Example) How was glycolysis discovered?
A: It was elucidated in the early 1900s by chemists studying fermentation in brewer’s yeast, following Eduard Buchner’s experiments showing that yeast cell extracts contained everything needed for fermentation in a test tube.
Q: What does glycolysis accomplish for the cell?
A: It generates a small amount of ATP under anaerobic conditions and produces pyruvate, the precursor to acetyl-CoA, lactate, or ethanol.
Q: What is the overall net reaction of glycolysis?
Glucose + 2 NAD⁺ + 2 ADP + 2 Pi ⇌ 2 Pyruvate + 2 NADH + 2 H⁺ + 2 ATP + 2 H₂O
Q: What are the key enzymes in glycolysis?
A:
Hexokinase: Catalyzes the first step; inhibited by glucose-6-phosphate.
Phosphofructokinase-1 (PFK-1): Allosterically activated by AMP and fructose-2,6-bisphosphate; inhibited by ATP and citrate.
Pyruvate kinase: Catalyzes the final step; activated by AMP and fructose-1,6-bisphosphate; inhibited by ATP and acetyl-CoA.
Q: (Example) What is an example of glycolysis in everyday biochemistry?
A: Glycolysis regulates blood glucose. Deficiency in glucokinase (a hexokinase-related enzyme) causes maturity-onset diabetes of the young (MODY2), due to inability of liver/pancreatic cells to phosphorylate glucose → high blood glucose.
Q: How many enzymatic reactions make up glycolysis?
A: 10 enzymatic reactions.
Q: What does the glycolytic pathway accomplish?
A: Converts one glucose molecule to two pyruvates in the cytosol, producing a net gain of 2 ATP.
Q: What happens to pyruvate after glycolysis under aerobic conditions?
A: Pyruvate enters mitochondria, oxidized to CO₂ and H₂O, producing 30 more ATP — total 32 ATP per glucose.
Q: (Figure 9.16 concept) What advantage does glycolysis have under anaerobic conditions?
A: Glycolysis can generate 2 ATP without oxygen — sustaining ATP production when O₂ is limited (e.g., during exercise).
Q: What is the energy yield comparison between aerobic and anaerobic metabolism?
A: Glycolysis alone (anaerobic): 2 ATP per glucose (≈6% yield).
Complete aerobic oxidation: 32 ATP per glucose.
Q: What provides most of the ATP in organisms?
A: Oxidation of pyruvate and acetyl-CoA in the mitochondria.
Q: What is the major energy source for ATP in animals?
A: Stored fats (fatty acids) → oxidized via fatty acid oxidation and acetyl-CoA metabolism in mitochondria.
Q: What conservation occurs in glycolysis?
A: The six carbons and six oxygens from glucose are conserved in the two pyruvate molecules produced.
Q: What do the 10 glycolytic reactions involve?
A: Enzyme-catalyzed phosphoryl transfers, isomerizations, aldol cleavage, oxidation, and dehydration—without net carbon or oxygen loss.
Q: What are the two stages of glycolysis?
Stage 1: ATP investment (reactions 1–5)
Stage 2: ATP earnings (reactions 6–10)
Q: What is required in Stage 1 of glycolysis?
A: Chemical energy input as phosphoryl transfer generates phosphorylated compounds for Stage 2.
Q: What enzyme catalyzes the first step of glycolysis?
A: Hexokinase (in all cells) phosphorylates glucose using ATP → glucose-6-phosphate (G6P).
Glucokinase (liver/pancreas) performs the same function.
Q: What happens to glucose-6-phosphate next?
A: It is isomerized by phosphoglucose isomerase to fructose-6-phosphate (F6P).
Q: What enzyme phosphorylates F6P?
A: Phosphofructokinase-1 (PFK-1) uses ATP to form fructose-1,6-bisphosphate (F1,6-BP).
Q: What happens in reaction 4 of glycolysis?
A: Aldolase cleaves F1,6-BP into two 3-carbon products:
Glyceraldehyde-3-phosphate (G3P)
Dihydroxyacetone phosphate (DHAP)
Q: What happens to DHAP?
A: It is isomerized by triose phosphate isomerase into another G3P molecule.
Q: What reaction begins Stage 2 of glycolysis?
A: Oxidation of glyceraldehyde-3-phosphate by G3P dehydrogenase (GAPDH), forming 1,3-bisphosphoglycerate.
Q: What is substrate-level phosphorylation?
A: Direct transfer of a phosphate group from a donor molecule to ADP → ATP.
Q: Where does substrate-level phosphorylation occur in glycolysis?
A:
Reaction 7: 1,3-BPG → 3-phosphoglycerate (enzyme: phosphoglycerate kinase) — first ATP yield step.
Reaction 10: Phosphoenolpyruvate → pyruvate (enzyme: pyruvate kinase) — second ATP yield step.
Q: What other reactions occur in Stage 2?
A:
Reaction 8: 3-phosphoglycerate → 2-phosphoglycerate (enzyme: phosphoglycerate mutase).
Reaction 9: 2-phosphoglycerate → phosphoenolpyruvate (enzyme: enolase).
Q: How many ATP are produced in glycolysis overall?
A: 4 total ATP formed − 2 invested = net gain of 2 ATP per glucose.
Q: Why is substrate-level phosphorylation distinct from oxidative phosphorylation?
A: It does not require O₂ or ATP synthase; ATP is generated directly from high-energy intermediates.
Q: Why is the energy yield from glycolysis under anaerobic conditions considered inefficient?
A: Glycolysis produces only 2 ATP per glucose under anaerobic conditions (≈6% of the 32 ATP possible under aerobic conditions). This low yield highlights that most cellular ATP comes from oxidation of pyruvate and acetyl-CoA in mitochondria through the citrate cycle and oxidative phosphorylation.
Q: What is the overall summary of glycolysis as a metabolic pathway?
A: Glycolysis is a universal 10-step pathway that converts glucose into two molecules of pyruvate, generating a net of 2 ATP and 2 NADH. It functions under both aerobic and anaerobic conditions, links to numerous metabolic pathways, and serves as the entry point for carbohydrate catabolism and energy production.
Q: How can the regulation of a metabolic pathway be understood?
A: By examining the free energy changes (ΔG°′ and ΔG) of each reaction, which identify the steps that drive the pathway toward product formation.
Q: What does analyzing ΔG°′ and ΔG reveal about glycolysis?
A: It reveals which reactions are irreversible under physiological conditions and which are near equilibrium.
Q: Which glycolytic reactions are irreversible under normal conditions?
A: The reactions catalyzed by hexokinase, phosphofructokinase-1 (PFK-1), and pyruvate kinase — these have large negative ΔG values.
Q: What is true of the other glycolytic reactions?
A: They have small free energy changes, meaning they are near equilibrium and reversible depending on metabolite concentrations.
Q: What are the two main tasks of Stage 1 (ATP Investment) in glycolysis?
A:
Use ATP to generate phosphorylated intermediates that are negatively charged and trapped inside the cell.
Split the six-carbon compound fructose-1,6-bisphosphate (F1,6-BP) into two three-carbon sugars (G3P and DHAP), which interconvert.
Q: What are the phosphorylated intermediates in Stage 1 precursors for?
A: They are precursors to high-energy compounds 1,3-bisphosphoglycerate and phosphoenolpyruvate, used in Stage 2 for substrate-level phosphorylation.
Q: What happens in Reaction 1 of glycolysis?
A: Glucose is phosphorylated at C-6 using ATP to form glucose-6-phosphate (G6P) — activating glucose for catabolism.
Q: What type of reaction is this, energetically speaking?
A: The first ATP investment step, where the energy from ATP hydrolysis is used to transfer a phosphate group.
Q: What role does Mg²⁺ play in the reaction catalyzed by hexokinase?
A: The ATP–Mg²⁺ complex is the true substrate, shielding negative charges and stabilizing the reaction transition state.
Q: Which two enzymes catalyze glucose phosphorylation, and where are they found?
A:
Hexokinase: present in all cells; broad substrate specificity (glucose, mannose, fructose).
Glucokinase: present only in liver and pancreatic cells; specific for glucose.
Q: How do hexokinase and glucokinase differ in regulation?
A:
Hexokinase: inhibited by product (G6P); stops when glycolytic flux is low.
Glucokinase: not inhibited by G6P and has a lower affinity for glucose — acts as a metabolic sensor of blood glucose levels.
Q: How does hexokinase bind glucose?
A: Through an induced-fit mechanism that excludes water and brings ATP’s phosphoryl group close to glucose’s C-6.
Q: What structural change occurs when glucose binds to hexokinase?
A: The enzyme undergoes a large conformational change, like jaws clamping down on the substrate.
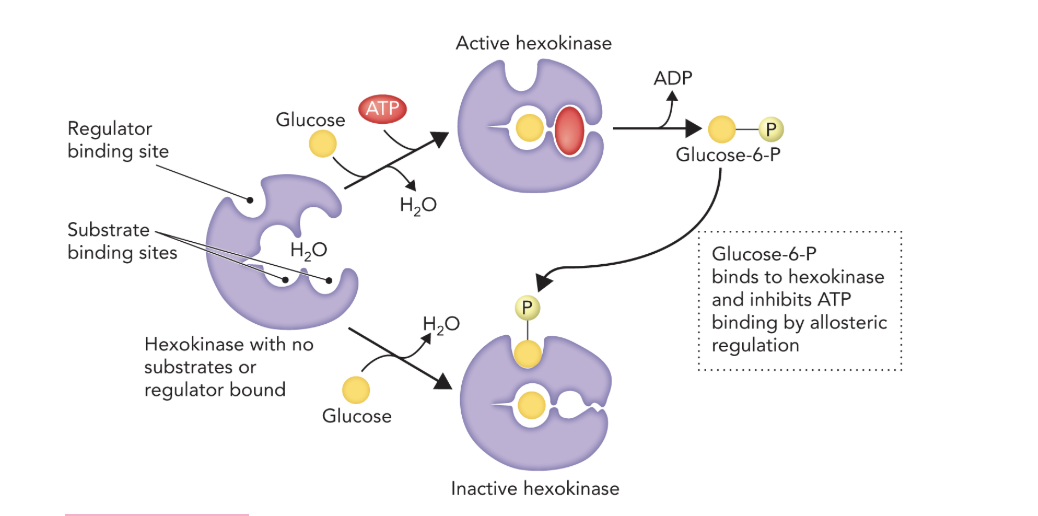
Q: What does Figure 9.21 illustrate?
A: It shows the induced-fit mechanism of hexokinase and how glucose-6-phosphate inhibits hexokinase by binding to its active site.
Q: What enzyme catalyzes the second reaction of glycolysis?
A: Phosphoglucoisomerase (also called phosphoglucose isomerase or phosphohexose isomerase).
Q: What does phosphoglucoisomerase do?
A: It interconverts an aldose (glucose-6-P) and a ketose (fructose-6-P) through opening and closing of the ring structure.
Q: What is the free energy change for this reaction, and what does it imply?
A: ΔG°′ = –2.9 kJ/mol, meaning the reaction is readily reversible and depends on intracellular concentrations of G6P and F6P.
Q: What structural change occurs in Reaction 2?
A: The six-membered ring of glucose-6-P is converted to a five-membered ring of fructose-6-P.
Q: What happens in Reaction 3 of glycolysis?
A: PFK-1 catalyzes the transfer of a phosphate from ATP to F6P, forming fructose-1,6-bisphosphate (F1,6-BP).
Q: What type of step is Reaction 3?
A: The second ATP investment reaction and a major regulatory step in glycolysis.
Q: Why is reaction 3 irreversible?
A: It has a large negative free energy change (ΔG°′ = –18.8 kJ/mol).
Q: What is the difference between bisphosphate and diphosphate compounds?
A:
Bisphosphate: two phosphate groups on different carbons (e.g., F1,6-BP).
Diphosphate: two phosphates covalently linked (e.g., ADP).
Q: How is phosphofructokinase-1 regulated allosterically?
Activated by: AMP and ADP (low energy state).
Inhibited by: ATP (high energy state).
This ensures glycolysis runs only when energy is needed.
Q: How do the allosteric effectors of PFK-1 interact?
A: AMP, ADP, and ATP bind to the same site; AMP/ADP are positive effectors, ATP is negative.
Q: What reaction does aldolase catalyze?
A: The cleavage of fructose-1,6-BP into two 3-carbon products: glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP).
Q: What does the term “lysis” in glycolysis refer to?
A: The splitting of a 6-carbon sugar into two 3-carbon molecules — occurs in Reaction 4.
Q: What bonds are cleaved by aldolase?
A: The C-3 and C-4 bonds of fructose-1,6-BP.
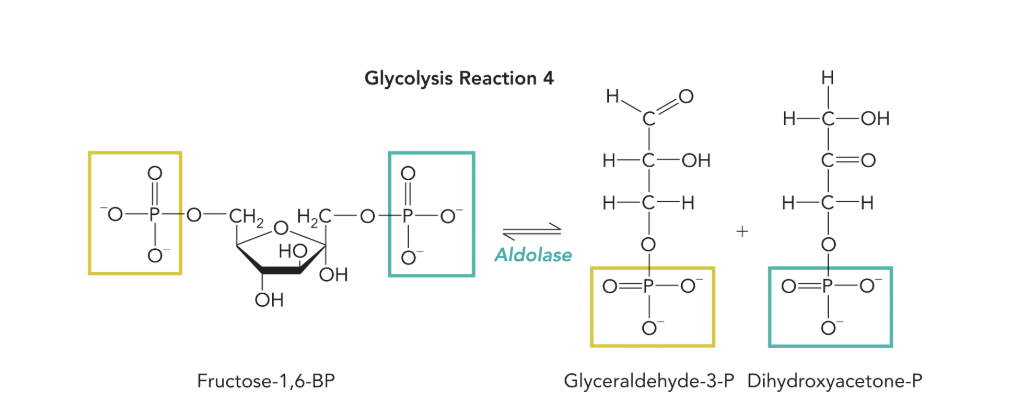
Q: What does Figure 9.24 show?
A: It depicts aldolase catalyzing the cleavage of F1,6-BP between C-3 and C-4, forming G3P and DHAP. Phosphate groups on C-1 and C-6 of F1,6-BP become the phosphates of the products.
Q: What type of reaction is catalyzed by aldolase mechanistically?
A: A reverse aldol condensation, involving formation of a Schiff base intermediate with a lysine residue in the active site.
Q: What are the four steps in the aldolase mechanism?
Fructose-1,6-BP binds to active site; lysine attacks the ketose carbon → Schiff base intermediate.
Base abstraction by an active site carboxyl group causes C–C cleavage → first product, G3P, released.
Isomerization forms a second Schiff base intermediate.
Hydrolysis releases the second product, DHAP.
Q: Why is examining the free energy (ΔG°′ and ΔG) of glycolytic reactions important for understanding regulation?
A: It identifies metabolic control points—reactions with large negative ΔG values (like those catalyzed by hexokinase, PFK-1, and pyruvate kinase). These steps drive the pathway forward and are targets for regulation, while reactions with small ΔG remain near equilibrium and reversible.
Q: What is the overall summary of Stage 1 (ATP investment phase) of glycolysis?
A: Stage 1 uses two ATP molecules to phosphorylate and trap glucose, forming fructose-1,6-bisphosphate, which is then split into two 3-carbon intermediates (G3P and DHAP). This primes the pathway for Stage 2’s energy-producing reactions, setting up substrate-level phosphorylation.
Q: What does the aldolase reaction illustrate about ΔG°′ and ΔG values?
A: Under standard conditions, aldol condensation is favored because the standard free energy change is highly positive (ΔG°′ = +23.8 kJ/mol). In the cell, metabolite concentrations favor cleavage, making the actual ΔG ≈ –0.4 kJ/mol, allowing the reaction to proceed during glycolysis.
Q: Why is Reaction 5 necessary?
A: Only glyceraldehyde-3-phosphate (G3P) can continue in glycolysis, so the enzyme triose phosphate isomerase (TPI) converts dihydroxyacetone-phosphate (DHAP) into G3P.
Q: How does Reaction 5 compare to Reaction 2?
A: It’s mechanistically similar but reversed — it converts a ketose (DHAP) into an aldose (G3P) instead of the other way around.
Q: What intermediate forms during Reaction 5?
A: The reaction proceeds through formation of an enediol intermediate (Figure 9.26).
Q: What is the outcome of Reaction 5 for glycolysis as a whole?
A: It completes Stage 1 of glycolysis — after investing 2 ATP, the pathway produces two molecules of G3P per glucose phosphorylated in Reaction 1.
Q: What are the four key features of Stage 2 reactions?
A:
Two G3P molecules enter Stage 2 for every glucose.
Two substrate-level phosphorylation reactions (by phosphoglycerate kinase and pyruvate kinase) produce 4 ATP total, giving a net 2 ATP gain per glucose.
Two NADH molecules form via glyceraldehyde-3-P dehydrogenase, later oxidized to generate more ATP through oxidative phosphorylation (or by lactate dehydrogenase anaerobically).
Reaction 10 is irreversible, ensuring pyruvate is the endpoint and preventing futile cycling with gluconeogenesis.
Q: Why are separate reactions required to reverse glycolysis in gluconeogenesis?
A: The irreversible steps (e.g., Reaction 10) must be bypassed using energetically costly reactions involving ATP and GTP to synthesize phosphoenolpyruvate from pyruvate.
Q: Why is the glyceraldehyde-3-phosphate dehydrogenase reaction critical?
A: It produces 1,3-bisphosphoglycerate (1,3-BPG), a high-energy intermediate used to drive ATP synthesis.
Q: What coupled redox events occur in Reaction 6?
A: NAD⁺ is reduced to NADH while G3P is oxidized, and the released energy drives addition of inorganic phosphate (Pᵢ) to form 1,3-BPG.
Q: Why must NAD⁺ be replenished?
A: Continuous glycolytic flux requires constant NAD⁺ supply — regenerated via the electron transport chain (aerobic) or lactate dehydrogenase (anaerobic).
Q: What are the four mechanistic steps of glyceraldehyde-3-P dehydrogenase?
A:
G3P binds; cysteine residue forms a thiohemiacetal intermediate.
Aldehyde oxidized; hydride transferred to NAD⁺, producing NADH + H⁺ and forming a high-energy acyl thioester intermediate.
NADH leaves; replaced by new NAD⁺.
The acyl thioester reacts with Pᵢ to form 1,3-BPG, regenerating the enzyme.
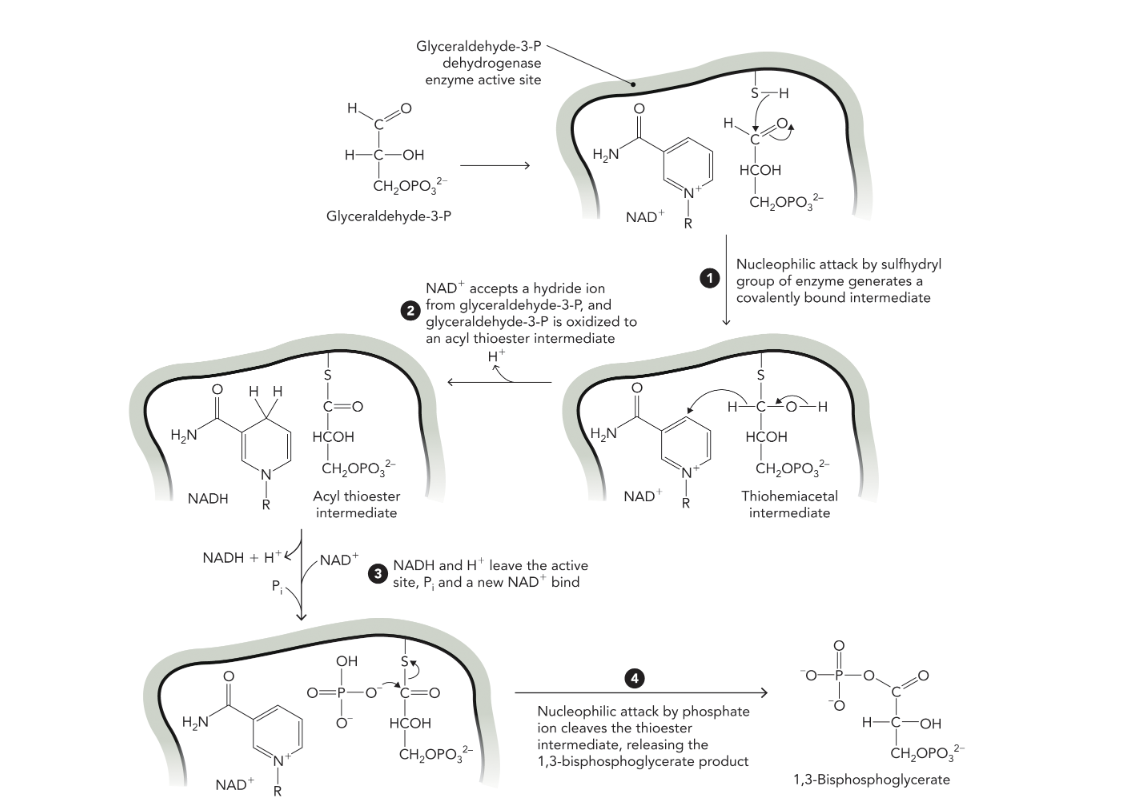
Q: What does Figure 9.28 illustrate?
A: The detailed four-step catalytic mechanism of glyceraldehyde-3-P dehydrogenase, including hydride transfer to NAD⁺ and regeneration of the active site.
Q: What free-energy change accompanies formation of 1,3-BPG?
A: The coupled oxidation-phosphorylation yields a standard ΔG°′ of –49.4 kJ/mol, more favorable than ATP hydrolysis (ΔG°′ = –30.5 kJ/mol).
Q: How is this free-energy difference used?
A: Phosphoglycerate kinase harnesses it in Reaction 7 to phosphorylate ADP, generating ATP via substrate-level phosphorylation.
Q: Why is the hydrolysis of some phosphate compounds (like 1,3-BPG and PEP) so exergonic?
A: Because their products (e.g., 3-phosphoglycerate) have greater resonance stabilization, favoring the hydrolyzed state.
Q: How does phosphoenolpyruvate (PEP) compare energetically to 1,3-BPG?
A: PEP has an even more favorable ΔG°′ (–61.9 kJ/mol). Its hydrolysis is driven by tautomerization of pyruvate (enol ↔ keto), which stabilizes the product and makes the forward reaction highly exergonic.
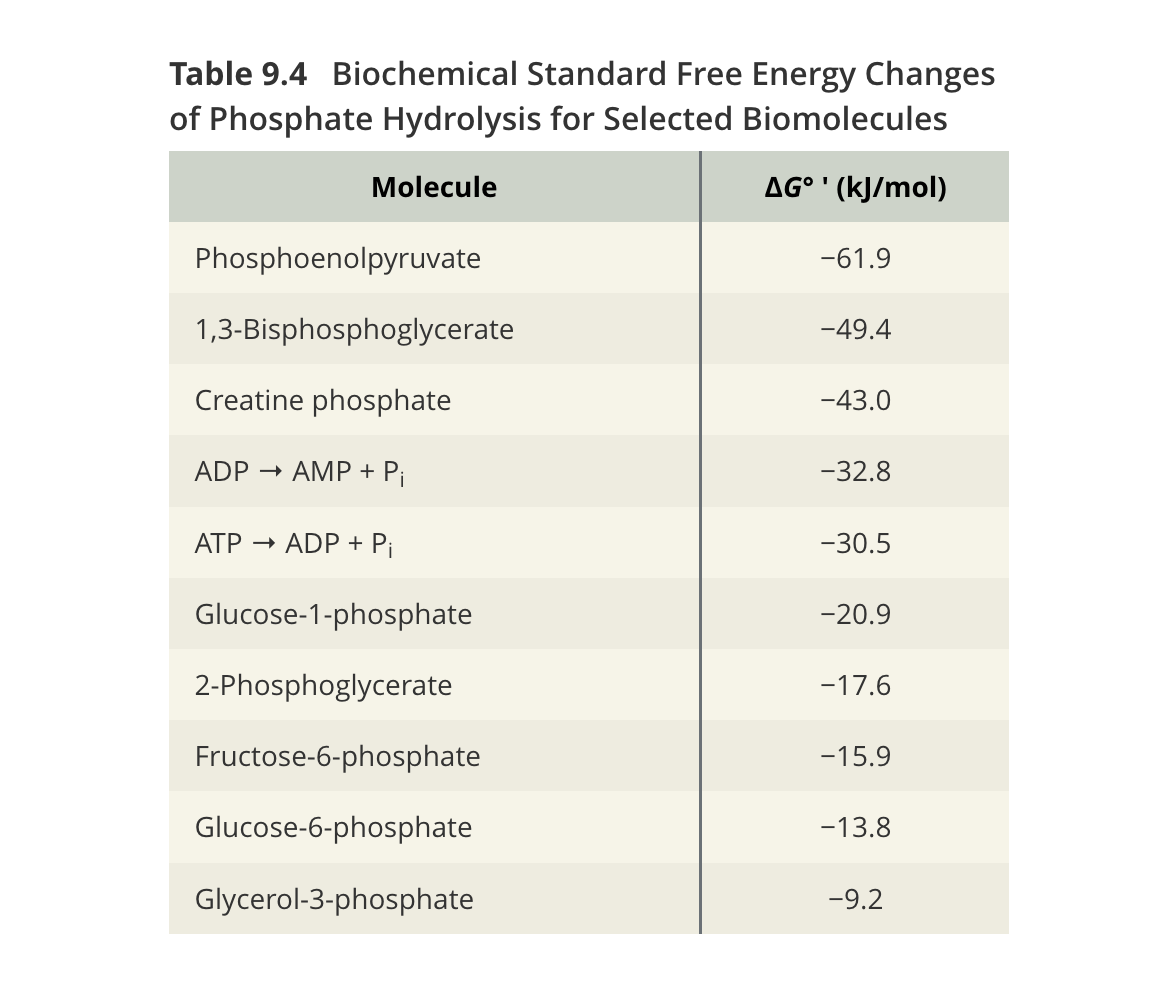
Q: What do the standard free energies in Table 9.4 reveal?
1,3-BPG (–49.4 kJ/mol) and PEP (–61.9 kJ/mol) > ATP (–30.5 kJ/mol) in energy yield.
Lower-energy phosphates like glucose-6-P (–13.8 kJ/mol) require ATP investment for phosphorylation in early glycolysis.
Q: What does phosphoglycerate kinase catalyze?
A: The conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate, transferring a phosphate to ADP to form ATP.
Q: Why is Reaction 7 known as the “payback reaction”?
A: It replaces the 2 ATP invested during Stage 1, restoring energy balance in glycolysis.
Q: How often does Reaction 7 occur per glucose molecule?
A: Twice — once for each G3P, producing 2 ATP total per glucose.
Q: What structural feature allows phosphoglycerate kinase to function?
A: It has two lobes, one binding ADP–Mg²⁺ and the other 1,3-BPG; substrate binding triggers a large conformational change that excludes water and aligns the phosphoryl group for transfer.
Q: How is the phosphoglycerate kinase mechanism similar to hexokinase?
A: Both use an induced-fit mechanism, bringing substrates together and excluding water to maintain a hydrophobic active site.
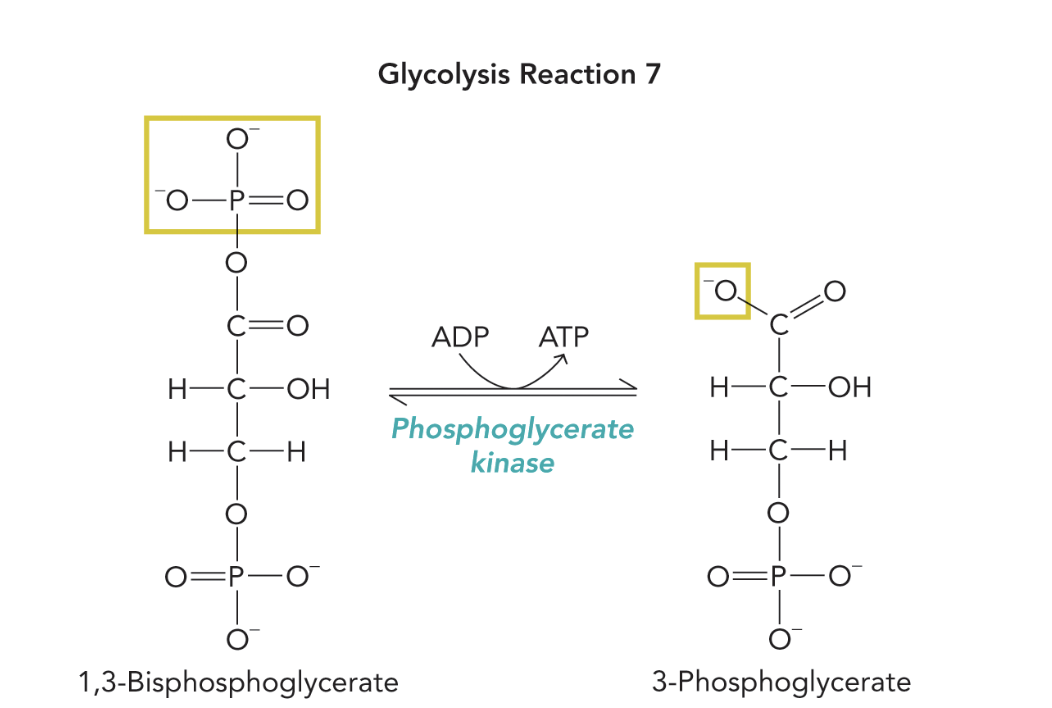
Q: What does Figure 9.29 show?
A: It depicts phosphoglycerate kinase catalyzing substrate-level phosphorylation, capturing the energy from 1,3-BPG to generate ATP and 3-phosphoglycerate.
Q: How do Stages 1 and 2 of glycolysis connect energetically?
A: Stage 1 invests 2 ATP to phosphorylate and split glucose into two G3P molecules, trapping energy in high-energy intermediates. Stage 2 then recovers this energy through oxidation and substrate-level phosphorylation, generating 4 ATP and 2 NADH, for a net gain of 2 ATP per glucose.
Q: How is energy flow coupled between Reactions 6 and 7 of glycolysis?
A: In Reaction 6, oxidation of G3P transfers electrons to NAD⁺ and forms 1,3-bisphosphoglycerate, storing energy in a high-energy phosphate bond. In Reaction 7, this stored energy is used by phosphoglycerate kinase to phosphorylate ADP → ATP, directly coupling oxidation to ATP synthesis.
Q: What are the actual free-energy changes for glycolytic reactions 6 and 7, and why are they significant?
A: The ΔG values are small (–1.3 kJ/mol and –3.4 kJ/mol), meaning both reactions are essentially reversible under cellular conditions.
Q: Which glycolytic reactions are irreversible, and why?
A: Reactions catalyzed by hexokinase, phosphofructokinase-1, and pyruvate kinase are irreversible due to large negative free-energy changes, making them key control points in metabolism.
Q: Why can’t small changes in metabolite concentrations reverse irreversible reactions?
A: Because the free-energy change is so unfavorable that physiological fluctuations in metabolite levels cannot shift equilibrium enough to drive reversal.
Q: How does gluconeogenesis overcome glycolysis’s irreversible steps?
A: It uses different enzymes in those steps to bypass the high-energy barriers and allow glucose synthesis from noncarbohydrate precursors.
Q: What important side reaction occurs in erythrocytes involving 1,3-bisphosphoglycerate?
A: It’s converted to 2,3-bisphosphoglycerate (2,3-BPG), an allosteric regulator of hemoglobin, linking glycolytic flux to oxygen transport.
Q: Which enzymes regulate this 2,3-BPG side pathway?
A:
Bisphosphoglycerate mutase: converts 1,3-BPG → 2,3-BPG.
2,3-BPG phosphatase: converts 2,3-BPG → 3-phosphoglycerate for re-entry into glycolysis.
Q: What is the physiological result of the 2,3-BPG shunt?
A: It creates a metabolic link between glycolysis and oxygen transport by modulating hemoglobin’s affinity for O₂.
Q: Why do defects in glycolytic enzymes affect oxygen transport?
A: Because 1,3-BPG and 2,3-BPG levels depend on glycolytic activity. Defects upstream reduce 2,3-BPG, while downstream defects increase it.
Q: How do changes in 2,3-BPG levels alter hemoglobin’s oxygen affinity?
:
↓ 2,3-BPG → curve shifts left → higher O₂ binding (R-state stabilization).
↑ 2,3-BPG → curve shifts right → lower O₂ binding (T-state stabilization).
Q: What broader concept does this illustrate?
A: How biochemical enzyme defects can cause seemingly unrelated physiological conditions, such as altered oxygen delivery.
Q: What is the purpose of Reaction 8 in glycolysis?
A: To convert 3-phosphoglycerate → 2-phosphoglycerate, forming the precursor for phosphoenolpyruvate in Reaction 9 and setting up for final ATP production.