Chapter 3: The Chemistry of Organic Molecules
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
Organic Molecules
always contain carbon and hydrogen,
always bonded covalently,
usually associated with living organisms
and are often quite large
inorganic molecules
molecules that do not contain carbon,
usually bonded ionically,
often associated with non living things
and always contain small amounts of atoms
4 types of biomolecules
carbohydrates, lipids, proteins, nucleic acids
structural carbohydrates
Both polymers of glucose, Cellulose in plant cell walls, Chitin in the cell walls of fungi and crustaceans, and peptidoglycan found in bacterial cell walls\
carbon atoms
6 atoms, 4 electrons on the outer ring, can bond with up to four other atoms often with other carbons
carbon skeleton
The chain of carbon atoms that forms the structural backbone of an organic molecule. This also determines the shape
functional groups
cluster of specific atoms that always react in the same way
Hydrophillic (polar)
soluble in water, likes it
hydrophobic, polar
insoluble in water, hates it
Isomers
Two different molecules that have the same chemical formula
Monomer
one subunit
Polymer
many monomers linked together
monomer of proteins
amino acids
monomer of carbohydrates
monosaccharides
monomer of lipids
glycerol and fatty acids
monomer of nucleic acids
nucleotides
dehydration reaction
A chemical reaction in which two molecules become covalently bonded to each other with the removal of a water molecule.
examples of dehydration synthesis
monosaccharides to disaccharides. amino acids joining via peptide bonds
Hydrolysis
the chemical breakdown of a compound due to reaction with the addition of water.
example of hydrolosis
digestion of starch into glucose monomers
Monosaccharides
Single sugar molecules
glucose, fructose, galactose, ribose, and deoxyribose
have a backbone of 3-7 carbons
Disaccharide
A double sugar, consisting of two monosaccharides joined by dehydration synthesis.
Lactose= Galactose+Glucose
Sucrose(table sugar)= Glucose+fructose
Maltose=Glucose+Glucose
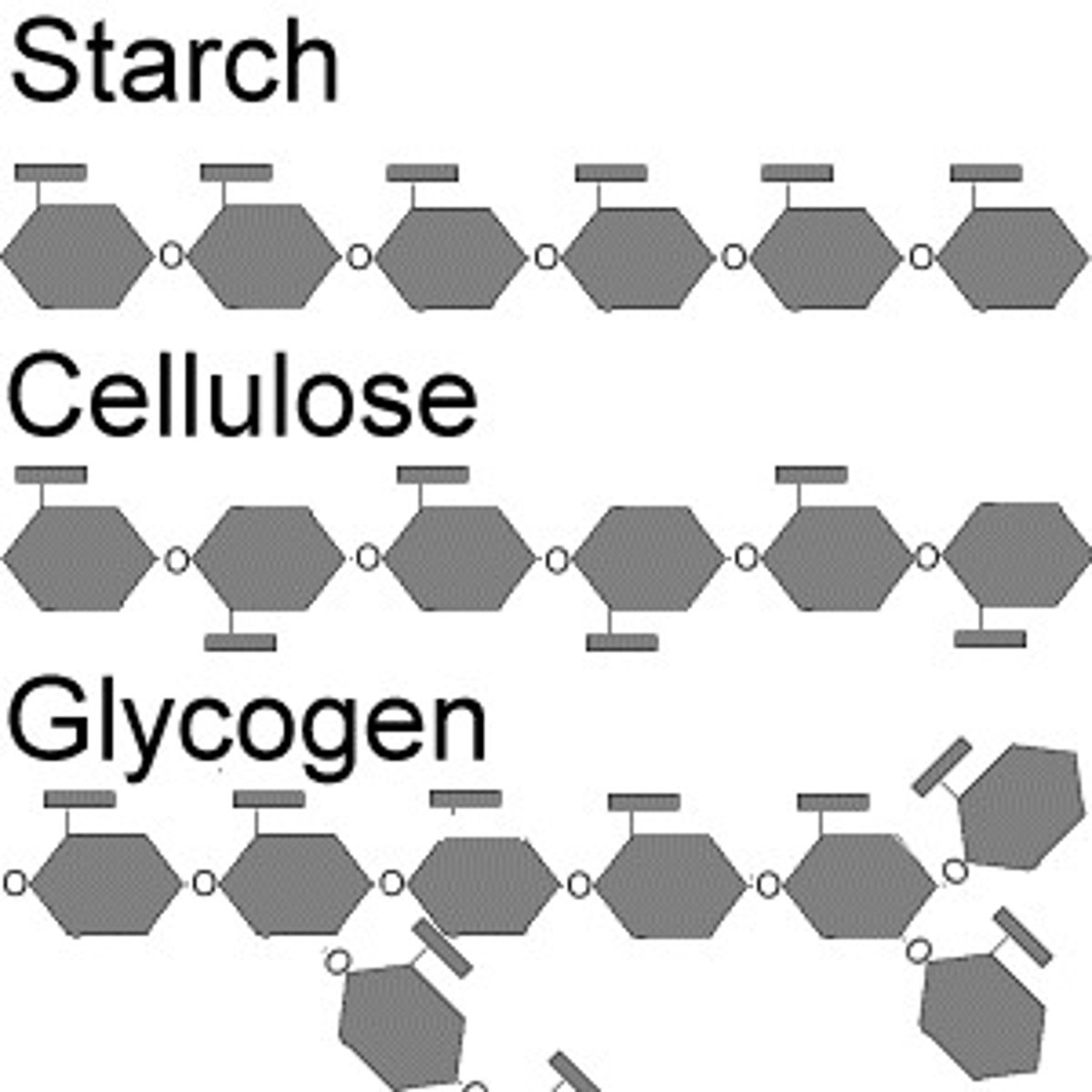
Polyssacharides
multiple sugars
Cellulose - in plants'
Chitin - in animals and fungi
Peptidoglycan - in bacteria
storage polysaccharides
starch (plants) used for short term energy storage, and glycogen (animals) found in the liver
Lipids
Energy-rich organic compounds, such as fats, oils, and phospholipids, that are made of carbon, hydrogen, and oxygen. nothing about them likes water
hydrocarbon
large nonpolar molecules
insoluble in water
Triglycerides
a long- term energy storage made up of a single molecule of glycerol and three molecules of fatty acid.
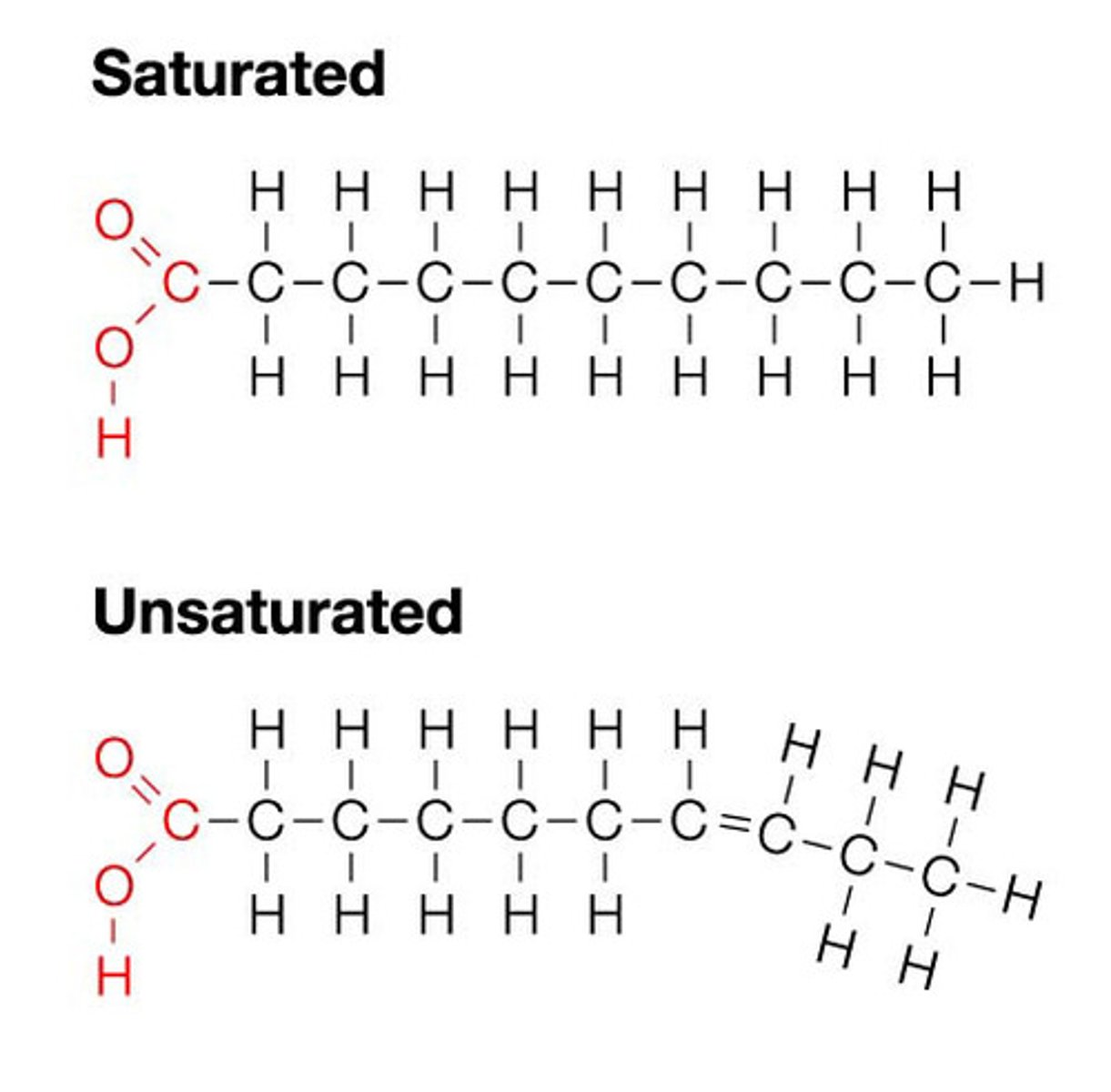
saturated fatty acid
no double bonds between carbon atoms
unsaturated fatty acid
1 or more double bond between carbons
Phospholipids (membrane components)
a lipid consisting of a glycerol bound to two fatty acids and a phosphate group. Heads are hydrophillic and tails are hydrophobic
proteins
extremely important to structure and function of cells
polymers of aa
polypeptide that has been folded into
a particular shape and has function
functions of proteins
structural support, enzymes, nutrient transport, defense, regulation, motion
How many amino acids are there that make up proteins?
20
How do amino acids differ?
have different R groups e.g. glycine has a hydrogen in its R group - simplest amino acid
peptide bond
The chemical bond that forms between the carboxyl group of one amino acid and the amino group of another amino acid
Dipeptide
Two amino acids bonded together
tripeptide
3 amino acids
Polypeptide
long chain of amino acids that makes proteins
How many polypeptide chains make up a protein?
Any amount
protein structure
must have correct shape to function
properly
native conformation
correct 3D shape; Held together with many hydrogen bonds (and maybe
some ionic and covalent bonds, too)
denatured
protein has lost its natural shape
Denatured proteins are not functional
main types of nucleic acids
DNA and RNA
Deoxtribonucleic acid (DNA)
stores genetic information, located in chromosomes and the nucleus
Robonucleic Acid (RNA)
serves primarily in assembly of proteins, found in nucleus, and cytoplasm
components of a nucleotide
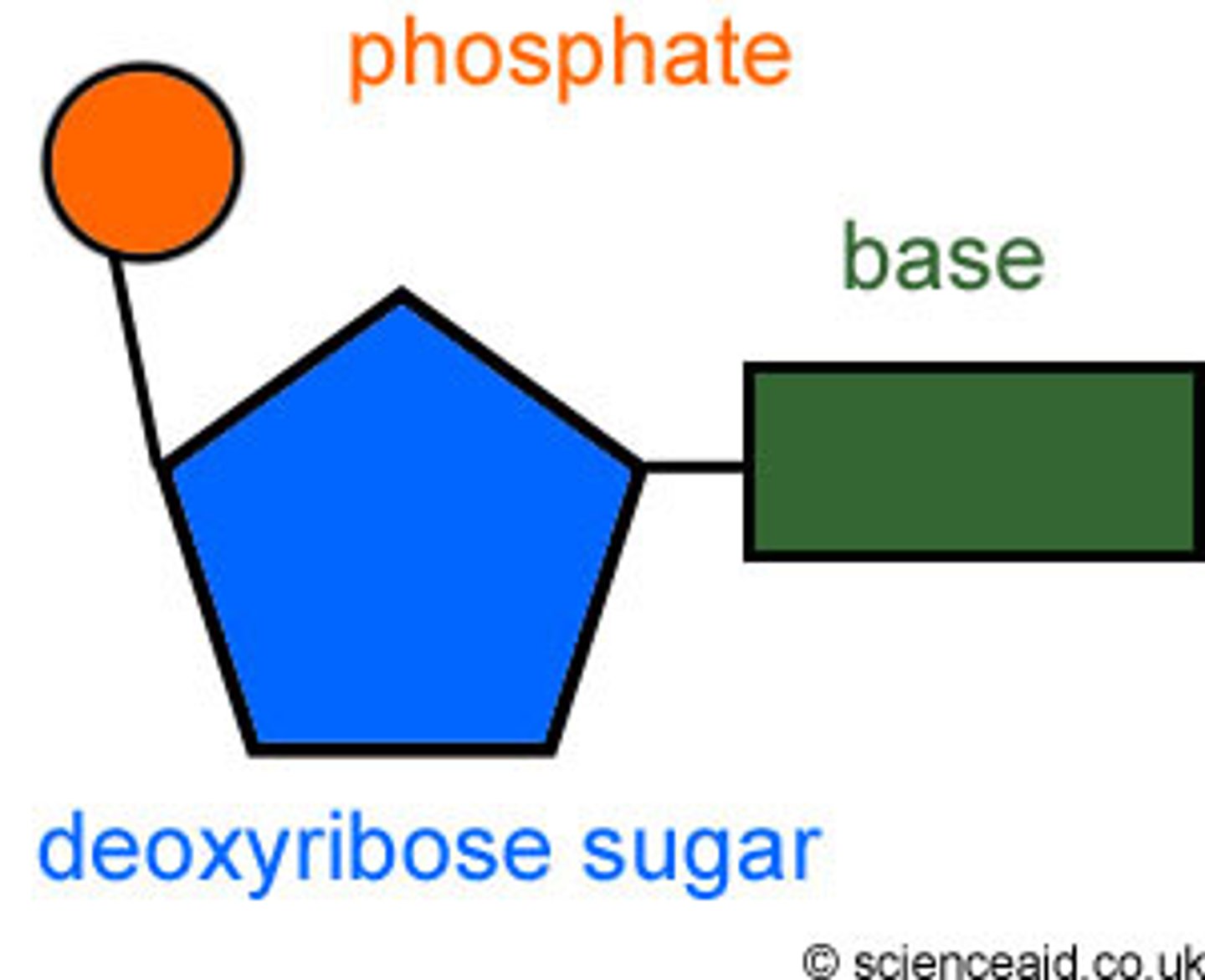
nitrogenous base, phosphate, pentose sugar
Pyrimadine bases
cytosine, thymine, uracil “CUT” (i could could my finger on the pyramid)
Purine bases
Adenine and Guanine
how do nucleotides join?
Covalent bonds btw the phosphate group on one and the ribose (sugar) of another.
DNA vs RNA structure
DNA: Double helix, adednine-thymine and guanine-cytosine
sugar is deocyribose
RNA: Single strand, Adenine-uracil and Guanine-cytosine. sugar is ribose
5’ - phosphate hanging off
3’ - no phosphate hanging off
ATP (adenosine triphosphate)
high-energy molecule
last 2 phosphate bonds unstable, easily broken (negatively charged, don’t like each other)
hydrolysis releases energy → used by cell for many things
organic molecules
Carbon-based molecules
carbohydrate
A simple or complex compound composed of carbon, oxygen and hydrogen.
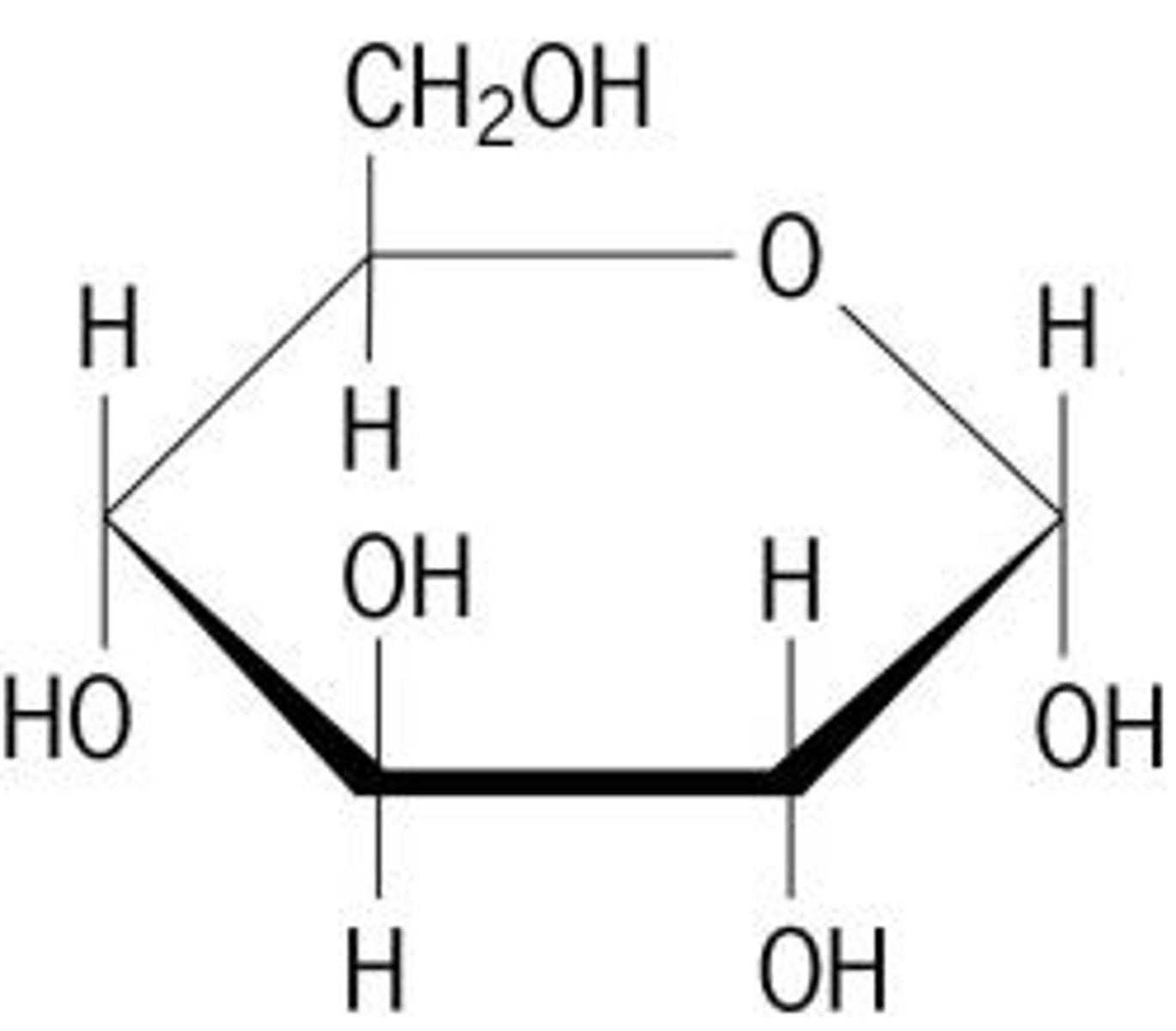
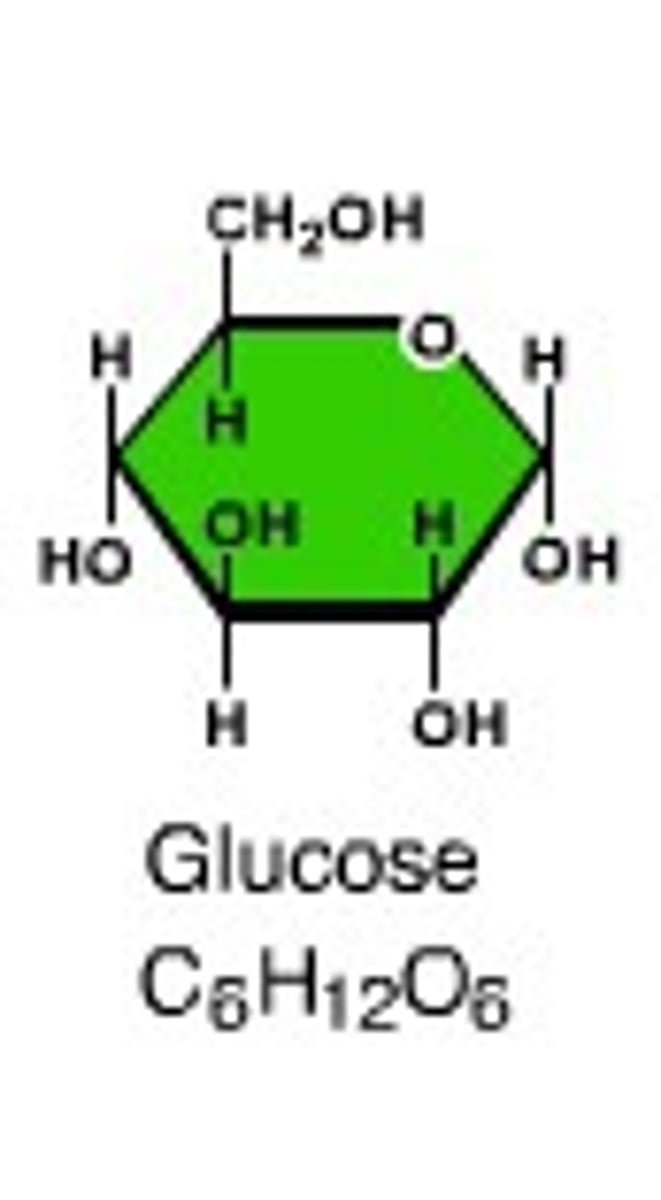
monosaccharide
monomer of carbohydrate, A single sugar molecule such as glucose or fructose, the simplest type of sugar.
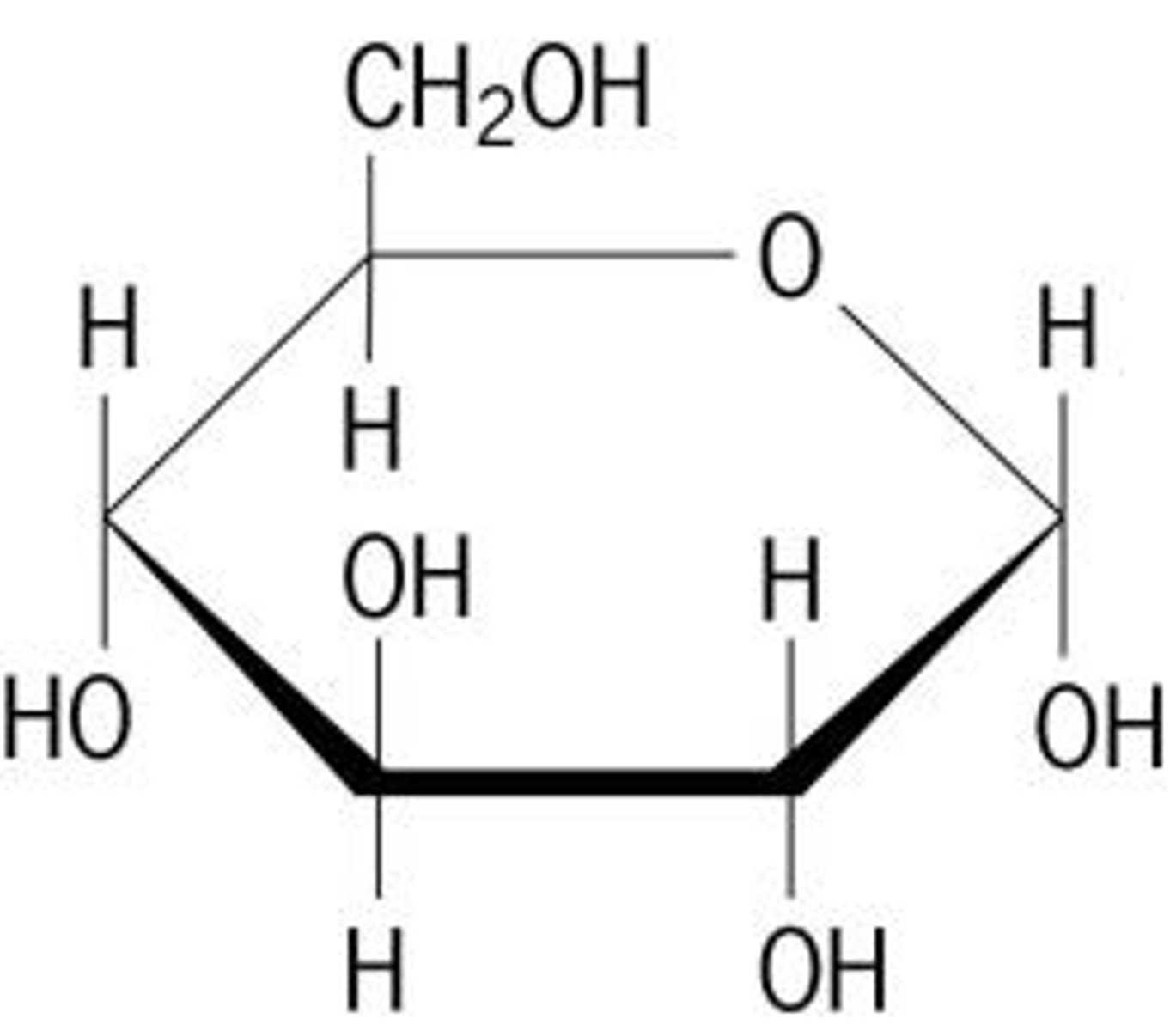
polysaccharide
Carbohydrates that are made up of more than two monosaccharides, polymer, aka complex carbohydrate
glucose
monomer, A simple sugar (monosaccharide) that is an important source of energy.
lipid
Fats, oils, and phospholipids, they are energy-rich organic compounds
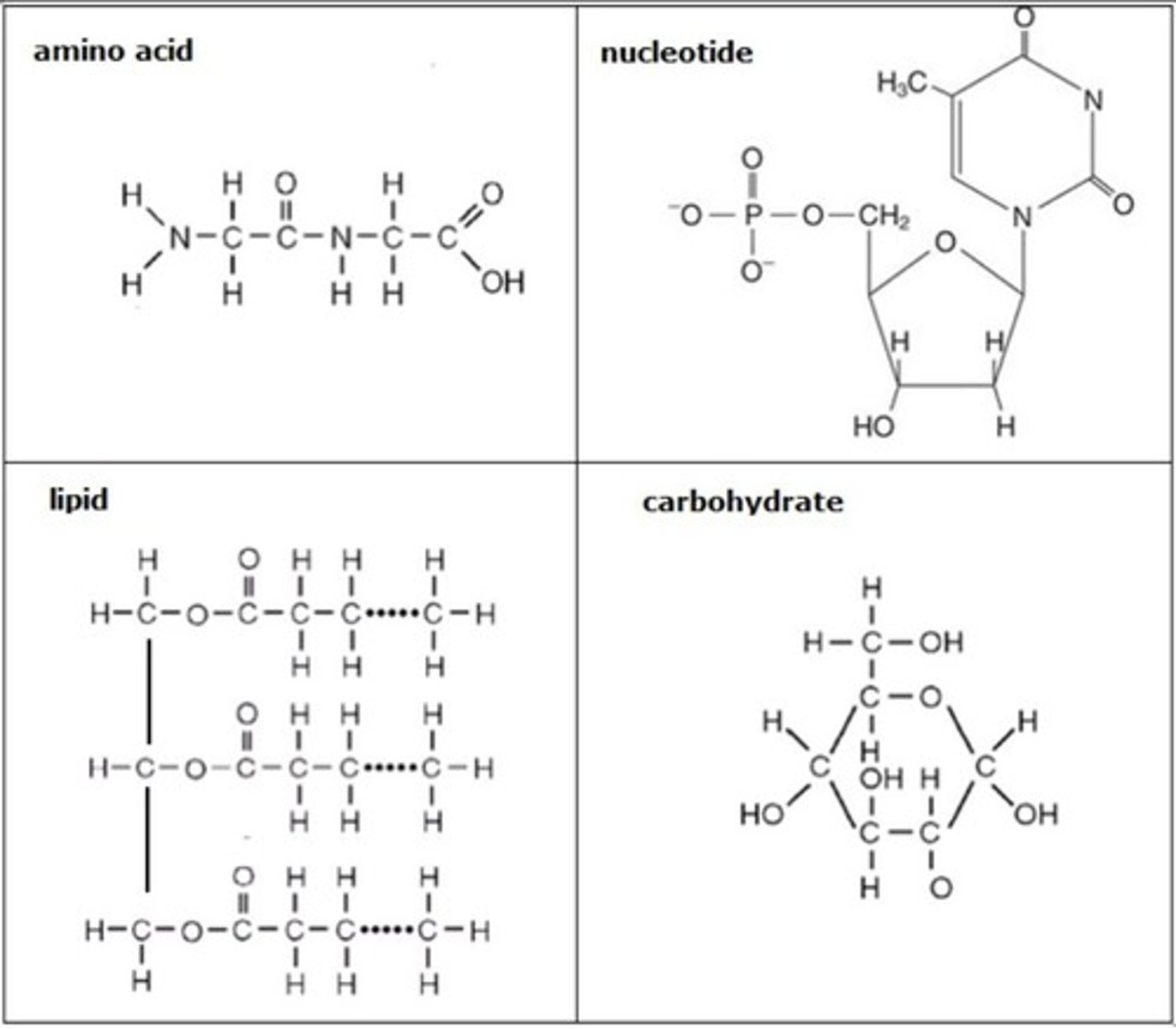
amino acid
monomer of proteins
protein
Chains of amino acids.
nucleotide
monomer of nucleic acid, A building block of DNA, consisting of a five-carbon sugar covalently bonded to a nitrogenous base and a phosphate group.