Homeostasis
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Homeostasis
The ability of the body to maintain a steady, but not static conditions in the internal environment, necessary for the health of all cells in the body.
How homeostasis is maintained
Biological systems use feedback loops to control homeostatic parameters
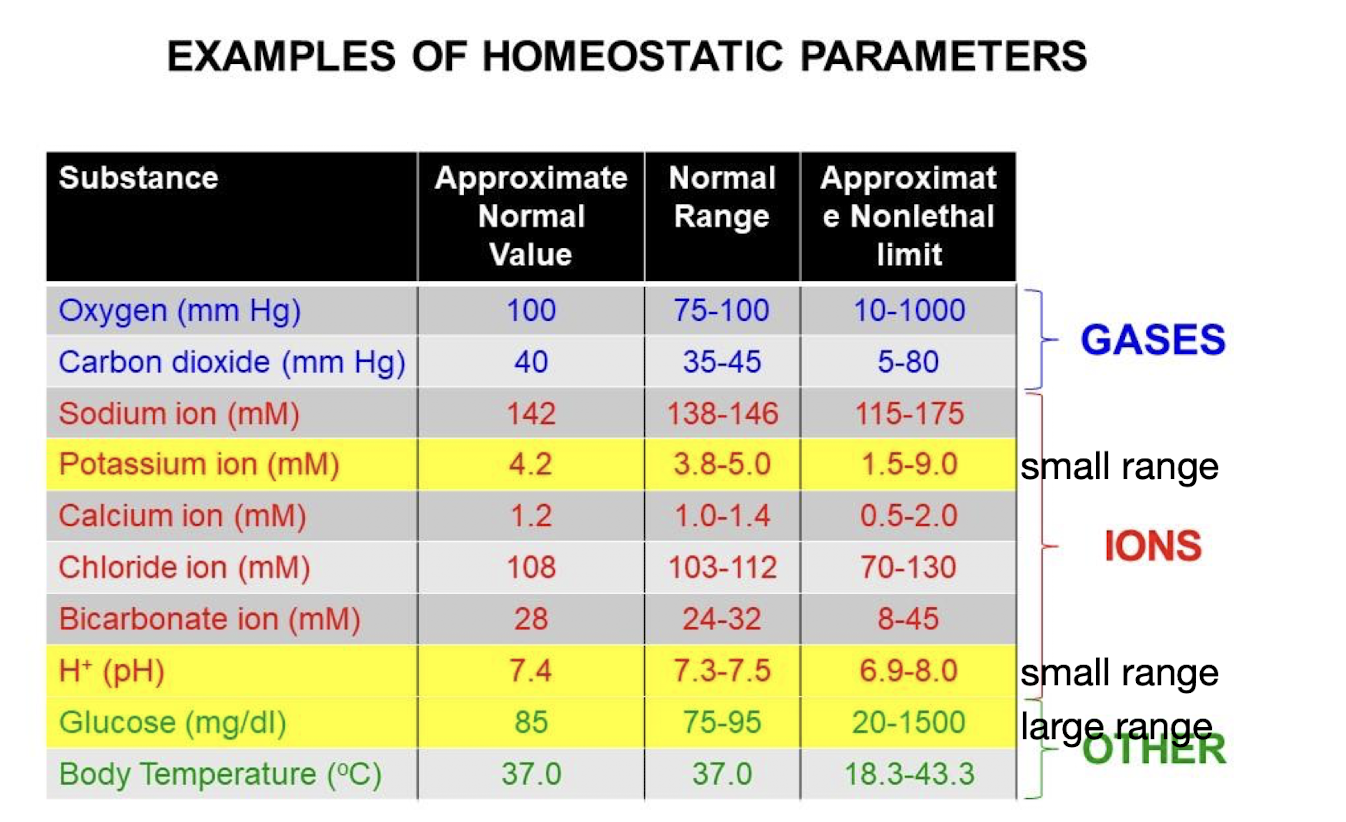
Homeostatic Parameters
O2, CO2, Sodium ions, Potassium ions, Calcium ions, Chloride ions, Bicarbonate ions, H+, Glucose, Body Temperature.
Some substances (Potassium/H+) are tightly regulated, while others (Glucose) are not so tightly regulated.
Negative Feedback Loop (Stable)
An initial increase in the variable leads to a subsequent decrease in the variable to bring it back to initial level. Physiologically, there are more of this type of feedback loops.
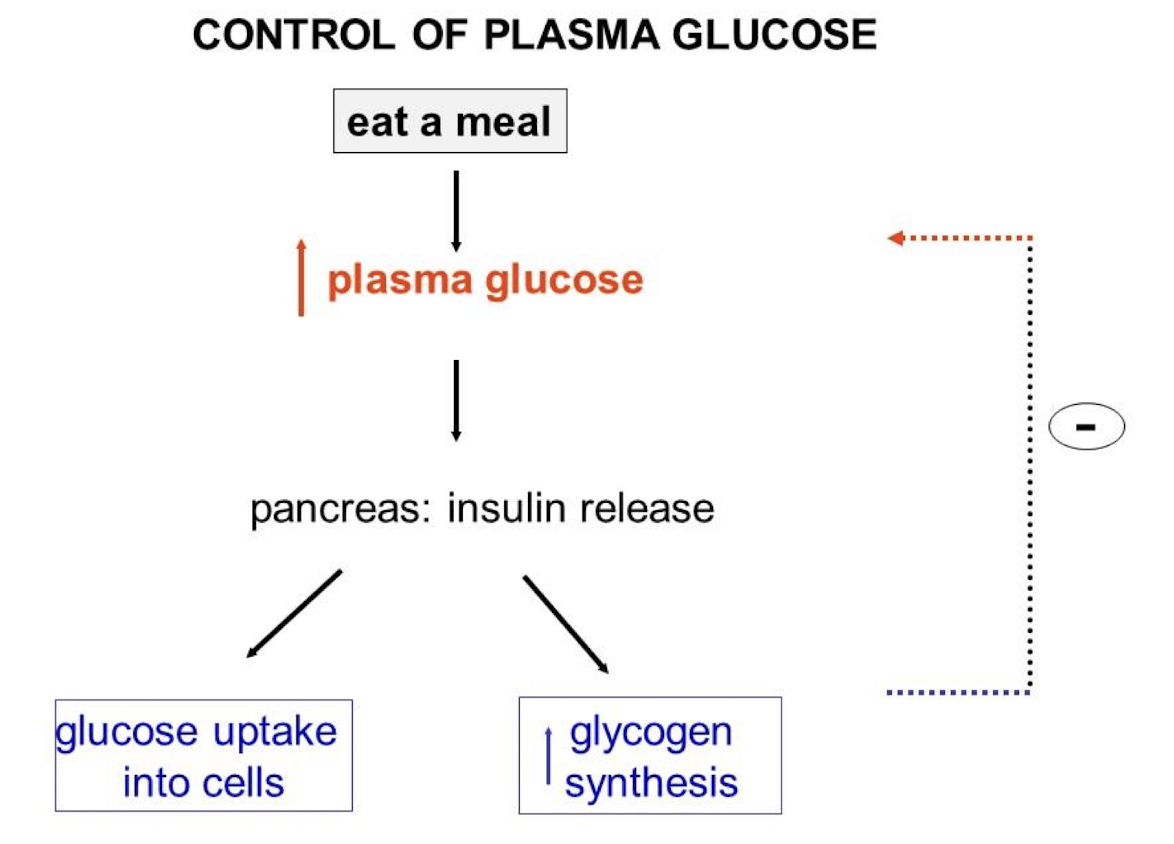
Ex. Control of Plasma Glucose
Positive Feedback Loop (Unstable)
An initial increase in the variable triggers a further increase in the variable.
Ex. LH secretion
Negative Feedback Loop Example (Glucose)
Ingest meal → increase in plasma glucose → triggers release of insulin → stimulates glucose uptake by cells (skeletal, adipose, and liver cells) & glycogen synthesis → plasma glucose back to basal level
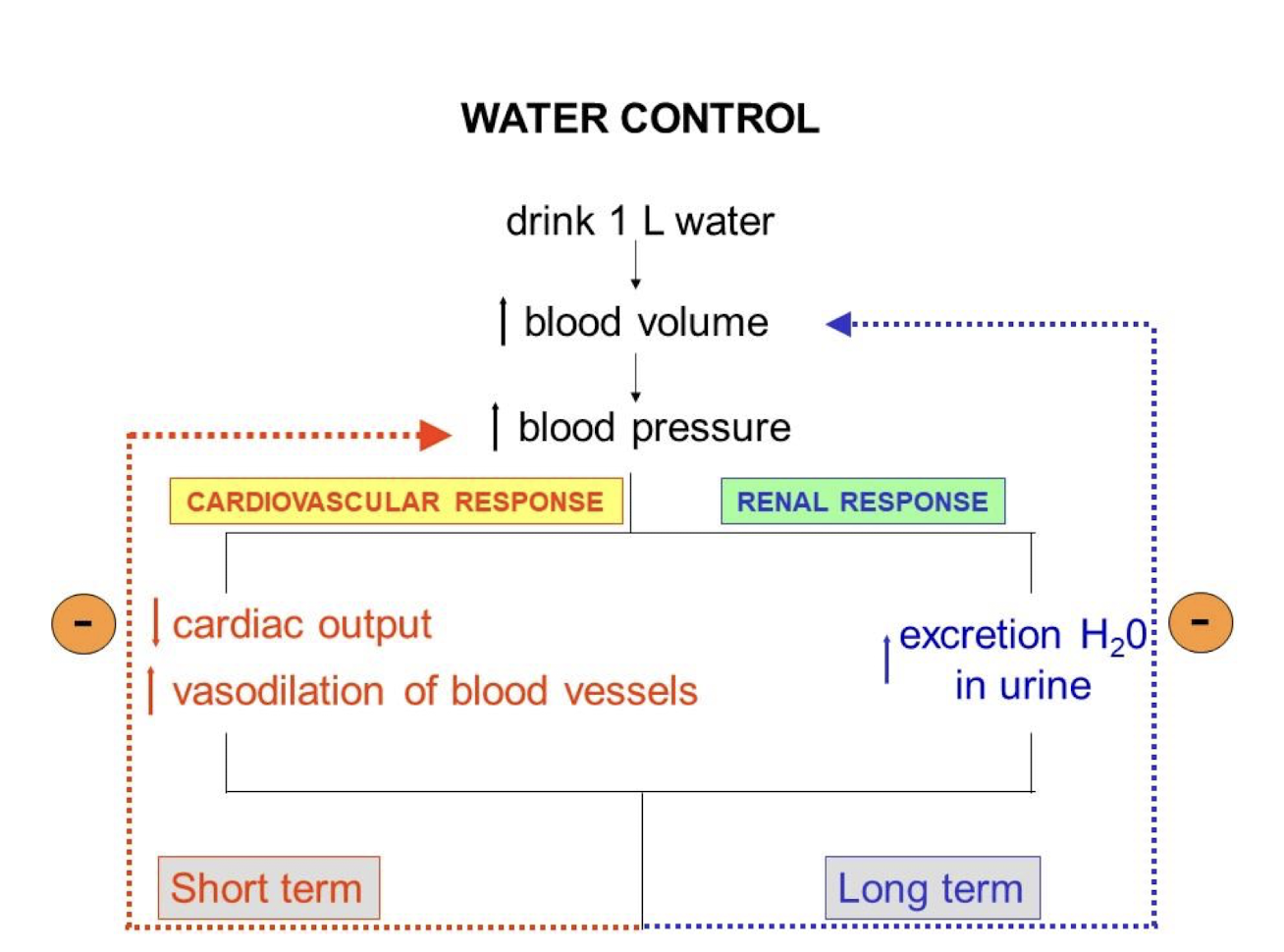
Negative Feedback Loop Example (Water Control)
Short Term:
Increase in volume of water in body → pressure sensors detect change in pressure due to said increase → triggers a decrease in cardiac output and an increase in blood vessel diameter → decreased volume of blood flows out of the heart → decreased blood pressure
Long Term:
Increase in volume of water in body → pressure sensors detect change in pressure due to said increase → triggers an increase in amount of water excreted by kidney → decreased blood pressure
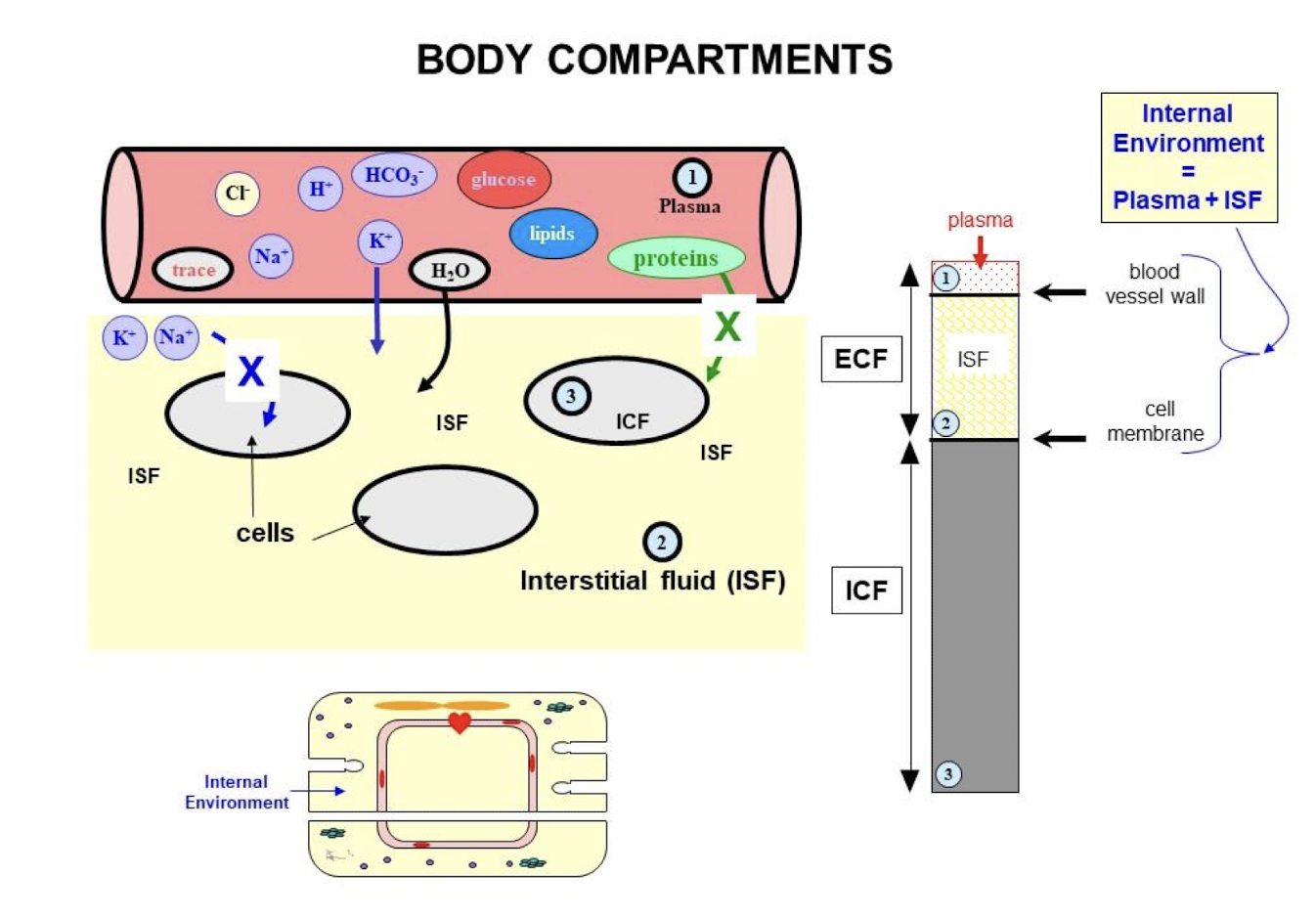
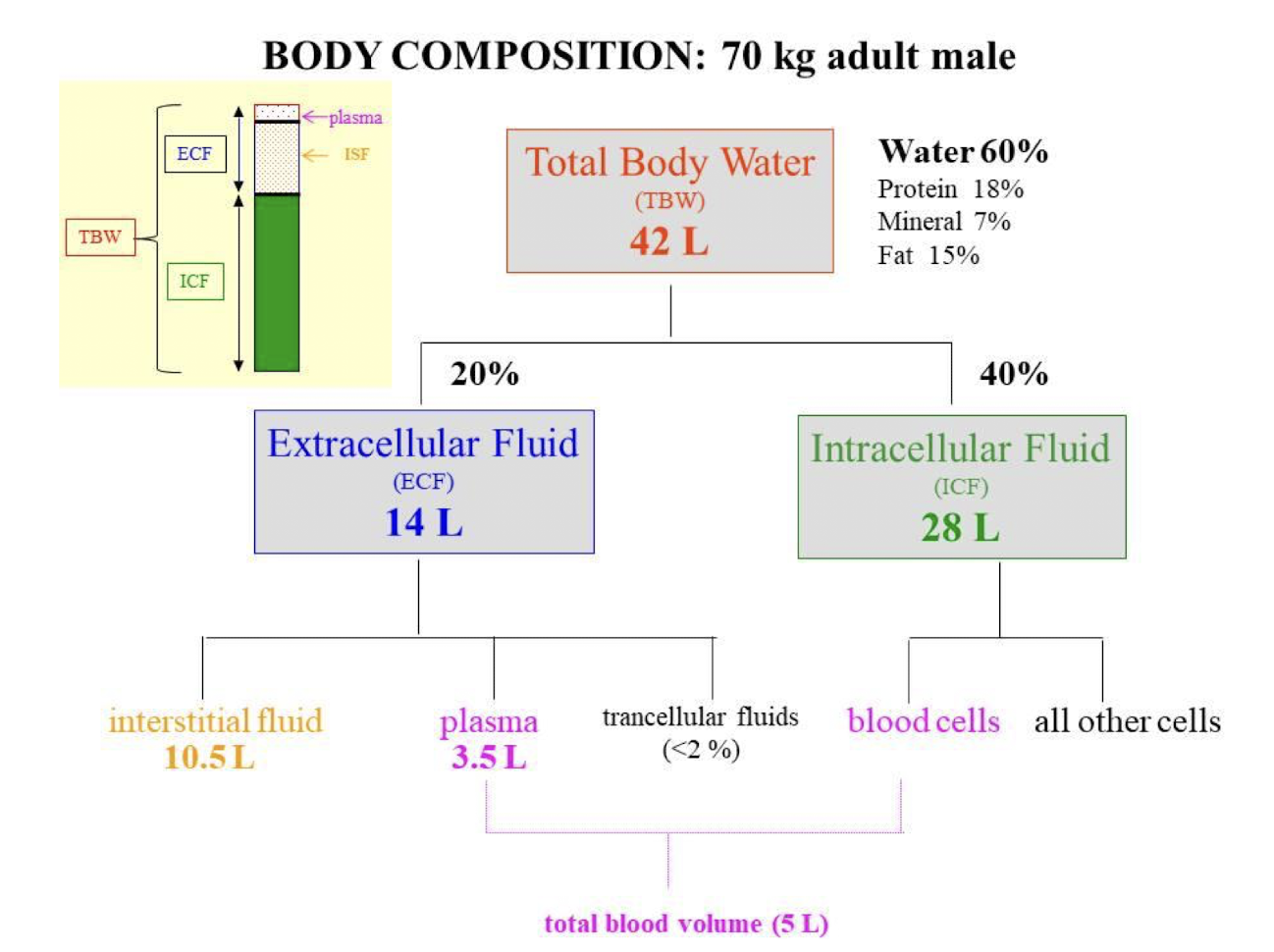
Plasma
Fluid and cells inside the circulation
Interstitial compartment (ISF)
Fluid surrounding all cells of the body
Intracellular compartment (ICF)
Fluid inside cells
Internal Environment / Extracellular Fluid (ECF)
Plasma + Interstitial compartment (ISF)
Barriers of Body Compartments
ICF separated from ECF by single lipid bilayer
Permeable to lipophilic substances (gases, hydrophobic hormones)
Impermeable to hydrophilic substances (glucose and ions)
ISF is separated from plasma by a blood vessel wall
Permeable to most small substances
Impermeable to large substances (large proteins, cells, and viruses)
Total Body Water (TBW)
ECF + ICF
Breakdown of Water Composition in a Human Body
TBW = 60%
ICF = 40%
ECF = 20%
Methods to Determine Body Compartment Volume
Estimate
Measure
Calculate
Estimation of Body Volumes
TBW = 0.6 x (body weight in Kg)
ICF = 0.4 x (body weight in Kg)
ECF = 0.2 x (body weight in Kg)
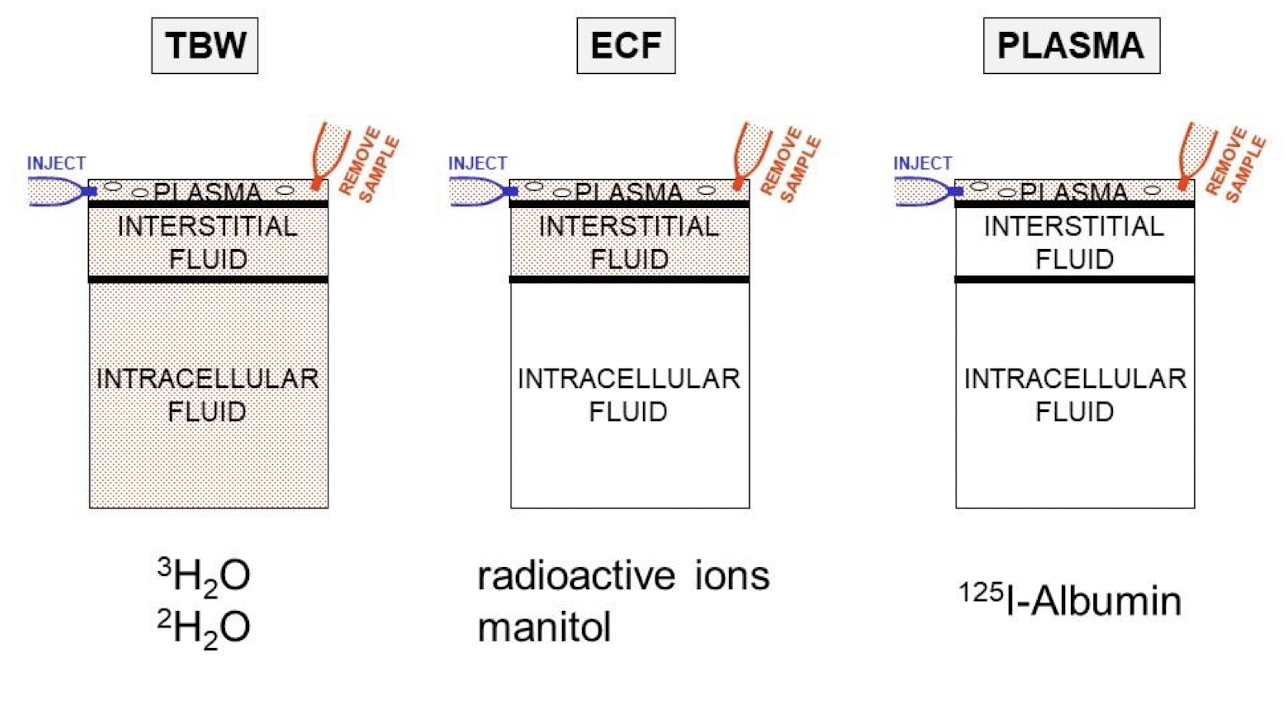
Measurement of Compartment Volumes
Inject a known amount of tracer (which will diffuse and restrict compartment being measured)
Sample is taken from compartment
When tracer is equilibrated:
Volume = (amount injected) / concentration
If some tracer is lost:
Volume = (amount injected - amount eliminated) / concentration
Intracellular volume calculation
Total body volume - Extracellular volume
Interstitial fluid volume calculation
Extracellular volume - Plasma volume
Blood volume (Vt) calculation
Plasma volume + Blood cell volume
Major extracellular electrolyte(s)
Na+, Cl-
Major intracellular electrolyte(s)
K+
Moles
Refers to the number of molecules of a given substance (1 mol = 6.022 x 10^23 particles)
Molarity
The concentration of a substance (1 M = 1 mol / L solvent)
Osmoles
The number of particles of a given substance
E. NaCl dissociates in aqueous solutions, yielding two osmoles
Osmolarity
The concentration of particles (1 OsMolar = 1 osmole / L solvent)
Equivalents
Refers to the number of particles of a given substance but takes into account the valence of a substance
Ex.
1 mol Na+ = 1 equivalent
1 mol Ca2+ = 2 equivalents
Molecular weight
The weight in grams of one mole of that substance
Plasma Osmolarity
Na+ is a major determinant. To account for pathologies such as diabetes and renal failure, use the equation [see image]
![<p>Na+ is a major determinant. To account for pathologies such as diabetes and renal failure, use the equation [see image]</p>](https://knowt-user-attachments.s3.amazonaws.com/efcca67a-3443-42b9-a203-9578f5ac7e10.png)
Percent Solution
The weight of the substance in that solution relative to the weight of water.
Calculating Osmolarity of a Percent Solution
How many Osmoles of NaCl in 2L of a 2.9% solution of NaCl?
2.9% = 2.9 / 100 = 0.029g of NaCl in 1mL
29g of NaCl in 1L
29g x (1 mol/58g) = 0.5 moles of NaCl per 1 L water
2 (# of particles that dissociate) x 0.5 moles = 1 Osmole of NaCl per 1L water
For 2L of water: 2 x 1 = 2 Osmoles of NaCl