Micro Metabolism
1/42
Earn XP
Description and Tags
Unit Metabolism for Micro 251 for exam 2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
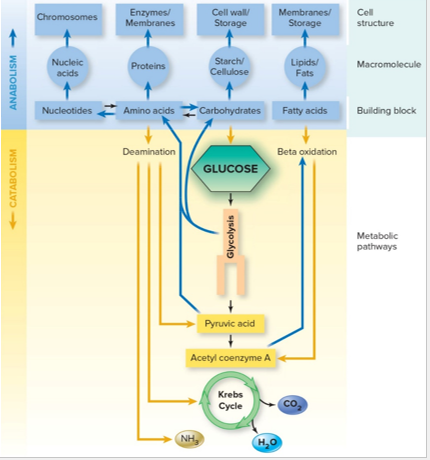
Metabolism
is the collective total of ALL chemical reactions that occur within the cell/organism
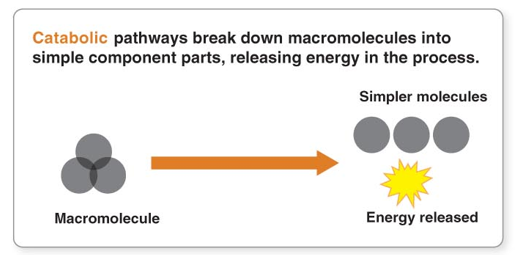
Catabolism
large molecules that are broken down into smaller once
Breaking down
Releases energy
Hydrolysis ( use water to break bonds)
Exergonic ( reactions produce more energy than consumed) (Releasing energy)
ex: Digestion of food, aerobic cell respiration
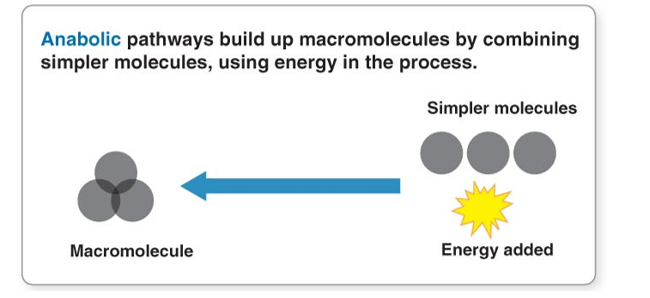
Anabolism
building up small molecules into Large Molecules
Building bonds
Absorbing energy
Dehydration synthesis (water is removed to create bonds)
Endergonic (building to supply energy ) ( “reactions "consume more energy than produced) (storing energy)
ex: Protein synthesis & DNA synthesis
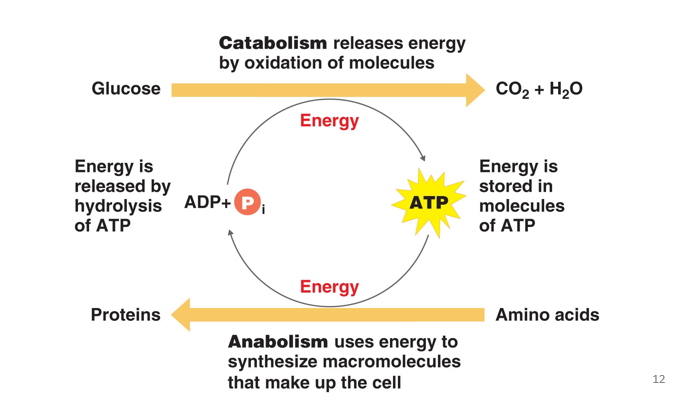
ATP
Is the energy carrier molecule within cell
Adenosine Triphosphate
energy is transferred from one component to another within the system
Energy currency
transferring energy
to and from reactions
** Anabolic and Catabolic reactions are “Coupled/Linked” by energy
Chemoheterotroph
use organic compounds as the carbon source and production of ATP
“Have to eat other things” (HETERO)
Ex: Animals, Fungi, Protozoa, and bacteria
Energy Source: Chemical
Carbon Source: Organic Compounds

Chemoautotroph
Oxidize inorganic molecules ( for energy ) to fix carbon dioxide into organic compounds
energy from inorganic compounds
carbon comes from Co2 Fixation
"Auto” - Self
Ex: Hydrogen - Sulfur, Iron, Nitrogen, carbon monoxide-oxidizing bacteria
Energy Source: Chemical
Carbon Source: Inorganic
Photoheterotroph
Use light (Sun) as source of energy to produce ATP and use organic compounds as carbon source
"Hetro” - eat other things
Ex: Green and purple non-sulfur bacteria, heliobacteria
Energy Source: Light
Carbon Source: Organic Compound
Photoautotroph
Use light as source of energy to fix carbon dioxide into organic compounds
Plants will collect energy from light (Sun)
Carbon from Co2 fix
ex: Cyanobacteria (group of photosynthetic bacteria that produce oxygen)
Ex: All plants, algae, cyanobacteria, and green and purple sulfur bacteria
Energy Source: Light
Carbon Source: Inorganic
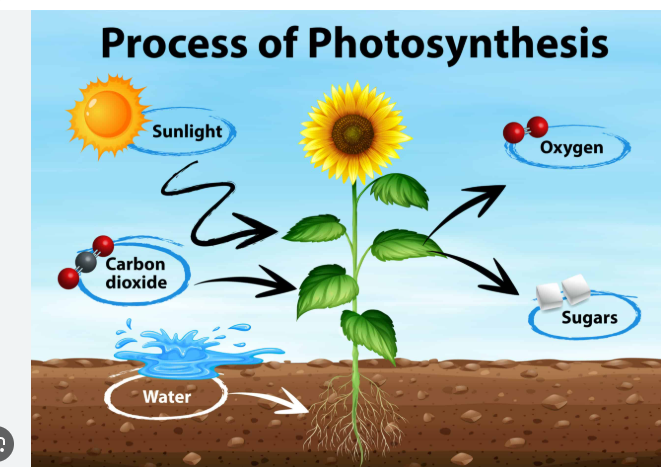
Photosynthesis
Metabolic process that uses light energy to fix inorganic carbon dioxide (CO2) into organic compounds ( sugar) This may produce oxygen gas (Depends on the photosynthetic pathway)
organisms that use it ex: plants, algae, and certain types of bacteria called cyanobacteria
Enzymes
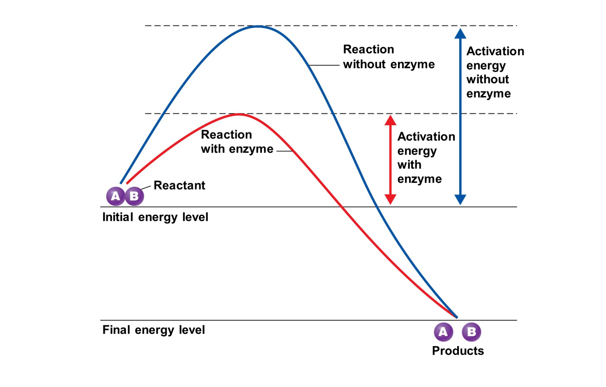
Is protein (macromolecule) that serves as a catalyst to “Speed up” chemical reactions by lowering activation energy for reaction to occur
Enzymes are specific for their substrates
Enzymes are sensitive to changes in their local environment (ph changes, temp, salinity)
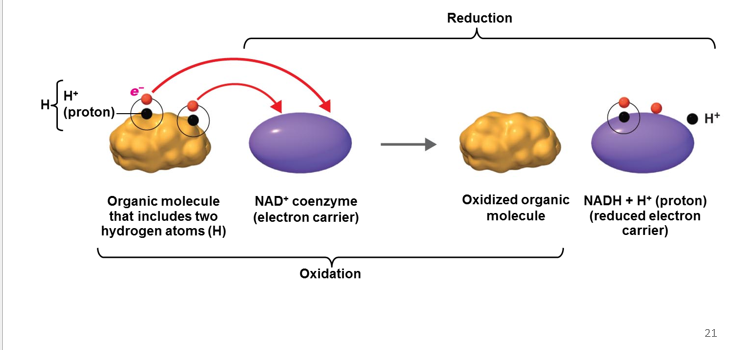
Reduction
is the gain of electrons. When something has undergone reduction, it means that it has gained electrons.
-The Gain of Electron
-Reducing agent - Gain of election
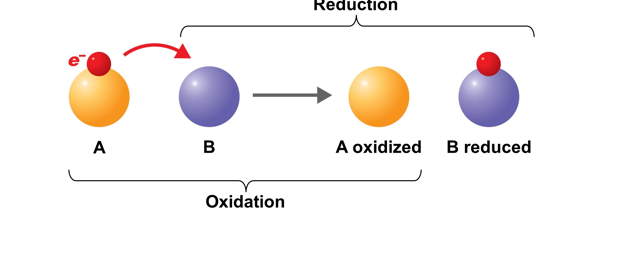
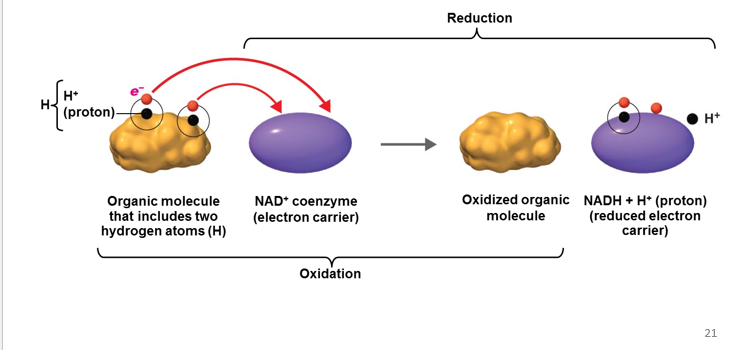
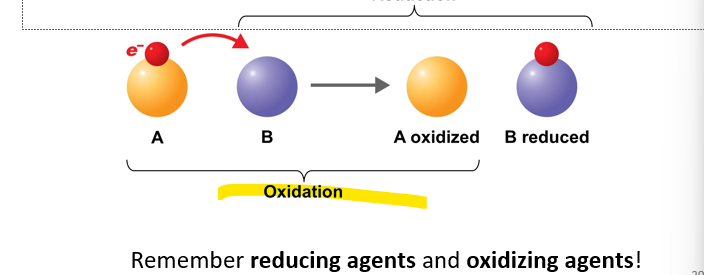
Oxidation
is the removal of electrons (e-) from an atom or molecule. When something has undergone oxidation, it means that it has lost electrons.
-The removal/lost of electron
-Oxidizing agents - lost of electron
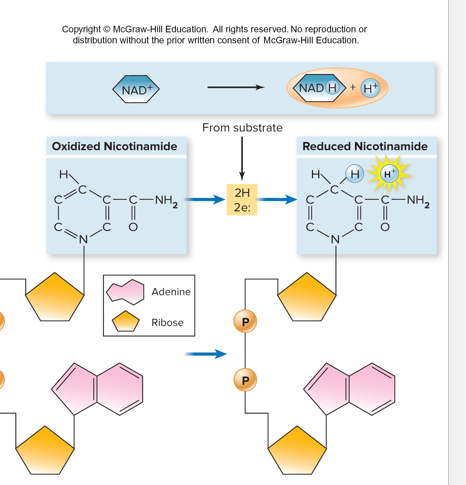
Catabolism Electron carrier Molecules:
NAD+/NADH
FAD/FADH
Anabolism Electron carrier Molecules:
NADP+/NADPH
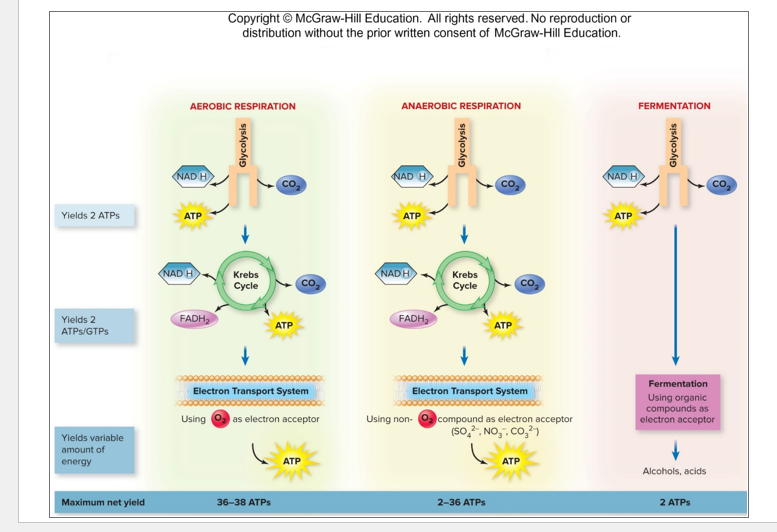
Microbes different ways to get ATP
Aerobic Respiration
Anaerobic Respiration
Fermentation
Lithotroph
Chemotroph that gets its energy from inorganic compounds (like reduced iron and H2s) Lithotrophy is only possible in microorganisms
Organotroph
Chemotroph that gets its energy from organic compounds, you think of this classification as chemoheterotroph
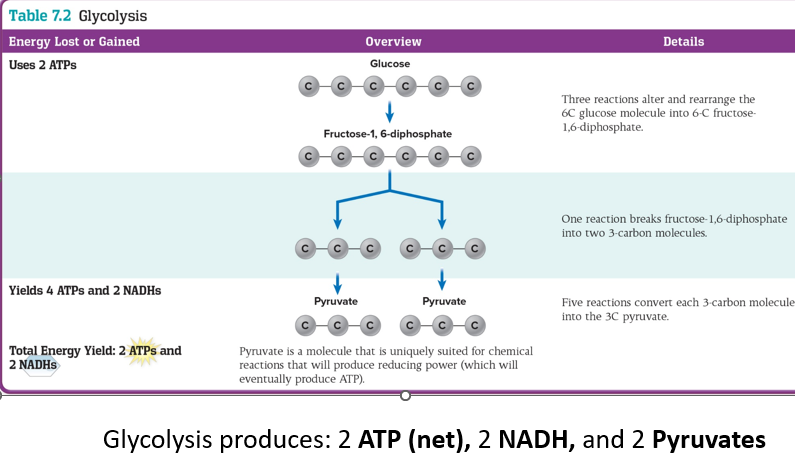
Carbohydrate Catabolism
The breakdown of sugar molecules to produce energy for cellular reactions
GLUCOSE is the most common carbohydrate for energy
(broken down for energy)
6 Carbon sugars has lots of energy in forms of electrons
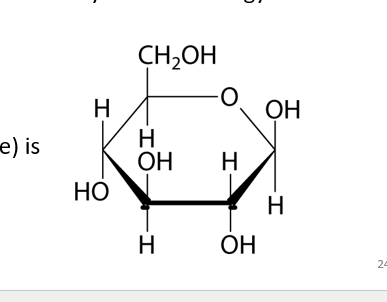
Embden-Meyerof-Parnas (EMP) Pathway
Most common type of Glycolysis
Generates: 2 ATP, 2 NADH, 2 Pyruvates
Found in Animals
2 Pathways called: Energy Investment & Energy payoff Phase
Type of Glycolysis pathway
Entner-Doudoroff (ED) Pathway
Some bacteria use this particular type of Glycolysis
Generates: 1 ATP, 1 NADH, 1 NADPH
-Originally used for anabolism ( may be older than EMP pathway in evolutionally history)
Commonly associated with Gram-Negative bacteria
Type of Glycolysis pathway
ATP produced by substrate-level phosphorylation
Means ATP is the primary energy carrier in biological system
a chemical reaction, without using oxygen or the electron transport chain.
Pentose Phosphate Pathway
Type of Glycolysis Pathway
used in Parallel with Glycolysis it generates 2 NADPH and ribulose-5-phosphate
used primarily for anabolism ( of nucleotides , amino acids, and fatty acids)
1st Intermediate in glycolysis
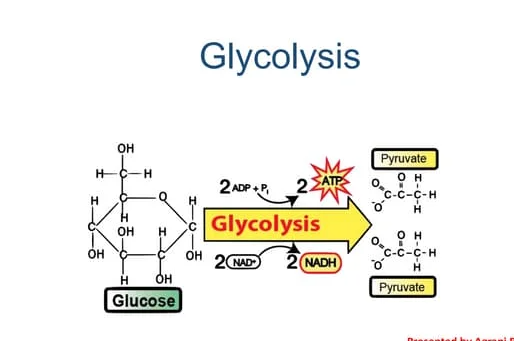
Glycolysis
Location: Cytoplasm of the cell
Oxygen requirement: Anaerobic (does not require oxygen)
Starting molecule: Glucose (6-carbon sugar)
End products:
2 pyruvate (3-carbon molecules)
2 ATP (net gain)
2 NADH (electron carriers)
** see the handout
Inorganic Molecule
is a molecule that does NOT contain BOTH carbon and hydrogen
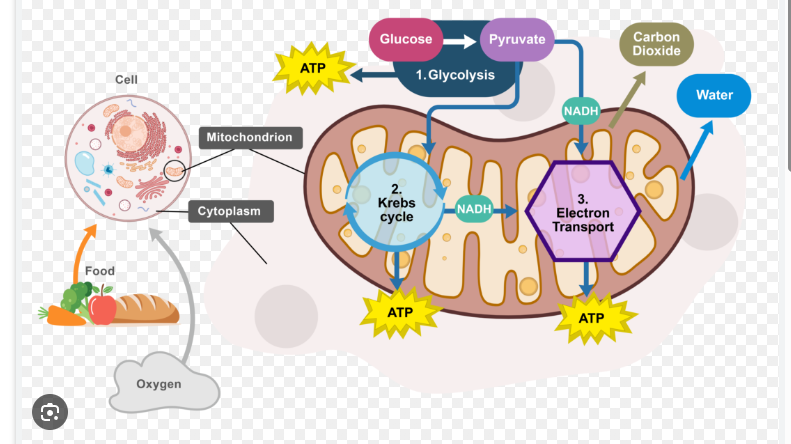
Stages of Aerobic Cellular Respiration
1) Glycolysis ( Split Glycose)
2) Pyruvate oxidation into acetyl-CoA (prepares it for Krebs)
3) Krebs Cycle ( More oxidation for energy)
4) Oxidative phosphorylation (electron transport chain and chemiosmosis) Oxygen is terminal electron acceptor
** see the handout
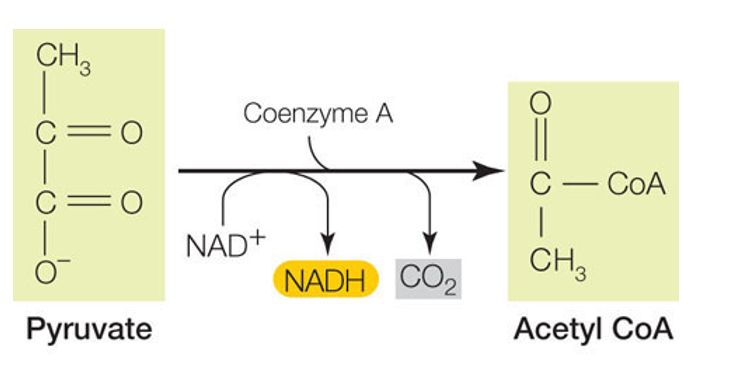
Pyruvate Oxidation
process of converting pyruvate (the end product of glycolysis) into acetyl-CoA, a molecule that enters the Krebs cycle.
It occurs in the cytoplasm
Products out ( Per Pyruvate) :
1 CO2
•1 NADH
•1 Acetyl CoA
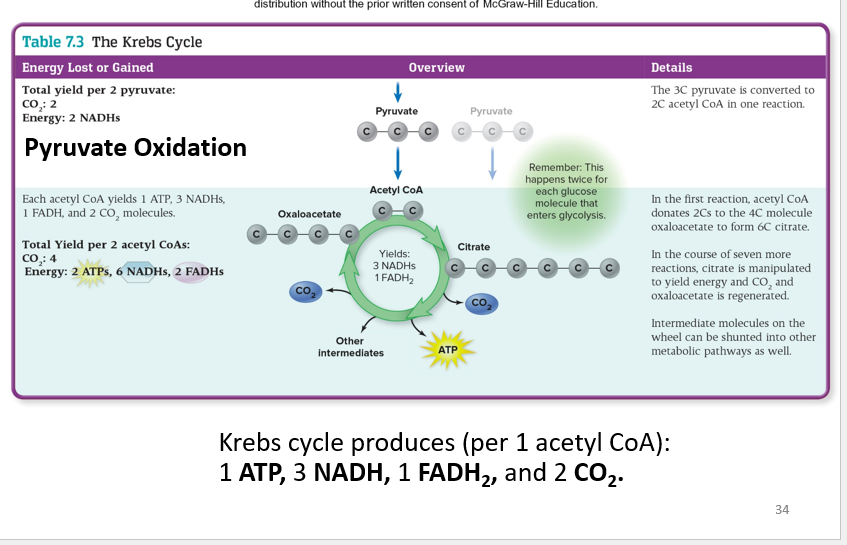
Citric Acid/ Krebs Cycle
Takes place in the Cytoplasm
Purpose: To extract high-energy electrons from carbon fuels (Acetyl-CoA)
Krebs cycle produce ( Per 1 Acetyl CoA)
1 ATP, 3 NADH, 1 FADH2 and 2 CO2
Oxidative Phosphorylation
Composed of two steps
-Electron Transport Chain
-Chemiosmosis
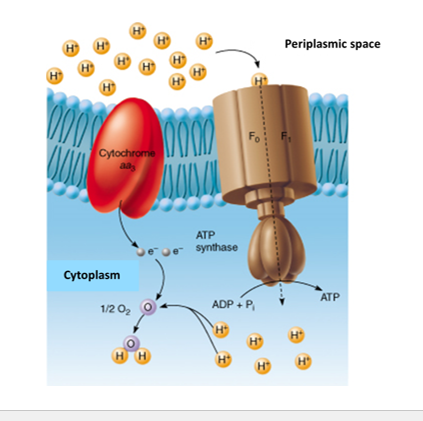
Electron Transport Chain
Located in the Plasma Membrane
Electron Carrier Molecule (NADH and FADH)
transfer their electrons to a series of proteins embedded in the cell membrane known as the ETC.
Oxygen is the Final electron acceptor and reduced to water
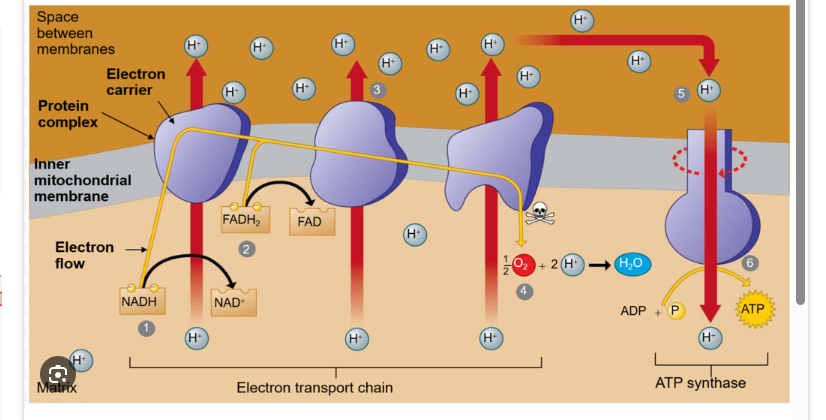
ETC Protons
Electrons pass through proteins in membrane
Hydrogen ions (H+) pumped out into (Periplasmic space)
Space between the plasma cell membrane and the cell wall.
Creating a Proton Gradient (Protons motive force)
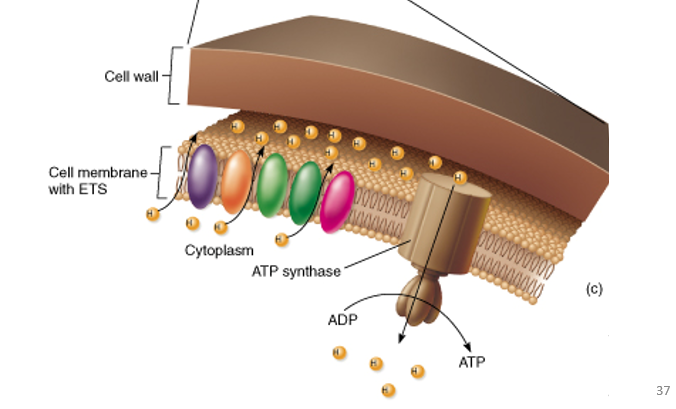
Chemiosmosis
Chemiosmosis is the process where ATP is made using the energy from a proton (H⁺) gradient across a membrane.
Protons (H⁺) flow down their concentration gradient through a protein called ATP synthase, which uses that energy to synthesize ATP.
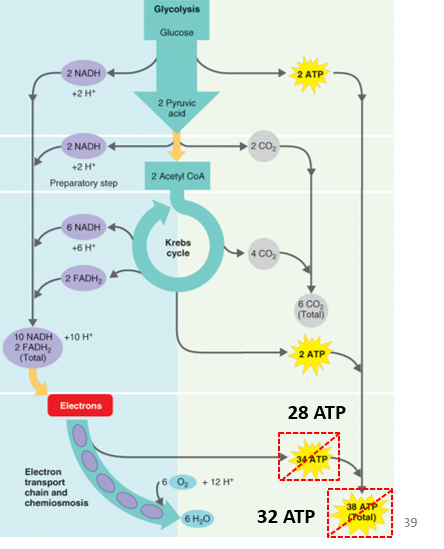
Aerobic Respiration Generate ATP # ?
-Regulate 32 ATP per glucose molecule
28 ATP is regulated from oxidative phosphate
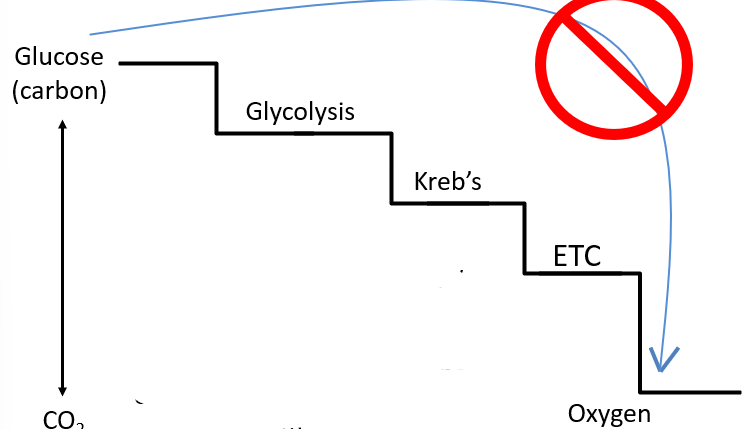
Important Step-Wise harvest in Energy
Important to have a Step-By Step Wise Harvest
Small steps to release energy from glucose
Releasing it all at once wont be efficient
Also help with easy regulated by the microorganism
Too much energy release if glucose oxidized all at once
2 Types of Cellular Respiration
Aerobic Cellular Respiration
Anaerobic Cellular Respiration
Both types use process called inorganic terminal electron acceptor and undergo similar steps
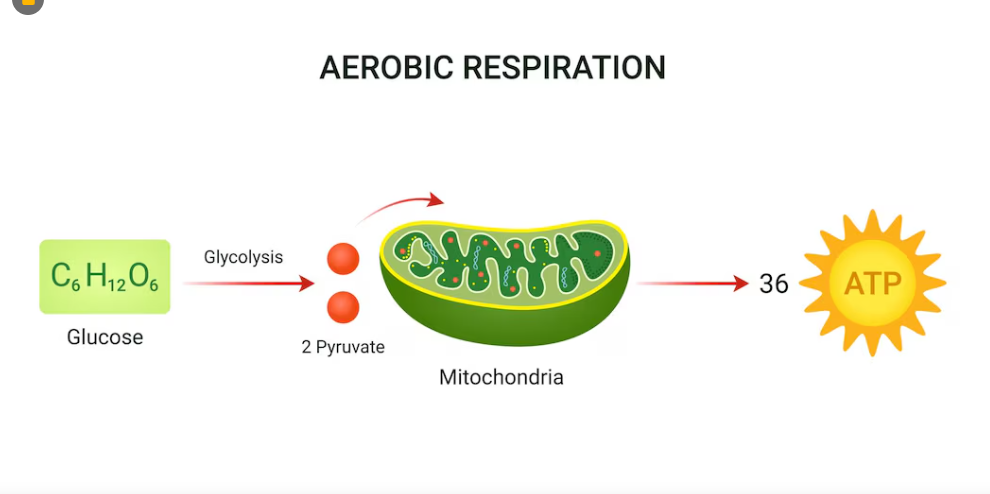
Aerobic Cellular Respiration
Uses oxygen as a terminal electron acceptor
-Oxygen is inorganic
Oxygen is Used
Aerobic Respiration - Electron Transport Chain
Anaerobic Cellular Respiration
An inorganic molecule than oxygen (like sulfate or nitrate) in used as terminal electron acceptors
No Oxygen is Used
Nitrate is Used
Anaerobic Respiration - ETC
Stages/Steps of Anaerobic
Glycolysis ( Split Glucose)
Pyruvate Oxidation into acetyl-CoA ( Prepares it for Krebs)
Krebs Cycle ( more oxidation for energy)
Oxidative Phosphorylation (ETC and Chemiosmosis)
Non-Oxygen inorganic molecules is terminal electron acceptor
** Anaerobic uses fewer ATP molecules per glucose than aerobic cellular respiration
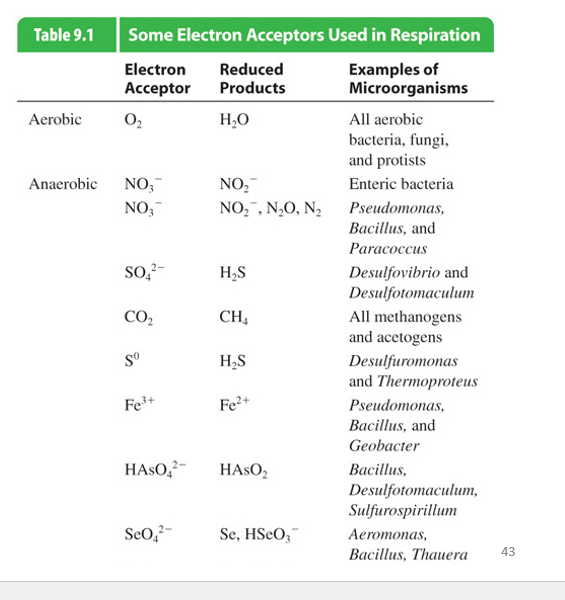
Inorganic molecules used in Anaerobic Respiration
Anaerobic:
Nitrate
Sulfate ion
Carbon Dioxide
Iron
hydrogen arsenate
Selenate ion
(Uses inorganic terminal electron acceptors)
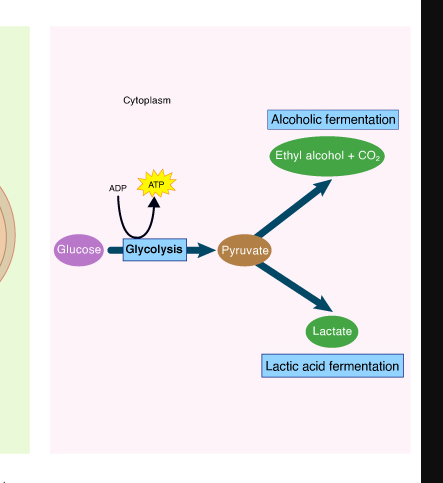
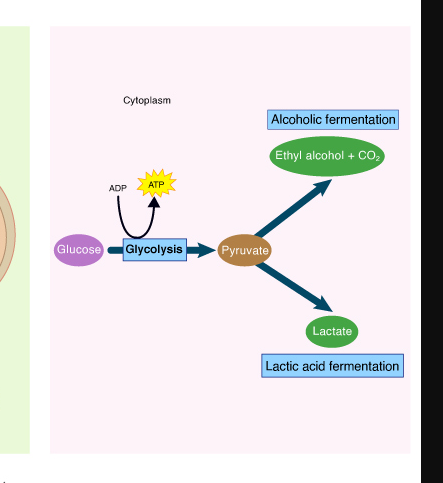
Fermentation
Is the incomplete oxidation of Glucose to generate ATP
Acceptor is organic
Does not require Oxygen
No oxidative phosphorylation
no ETC or Chemiosmosis is involved to generate ATP
Organic electron acceptors
** FERMENTATION IS NOT ANAEROBIC Respiration
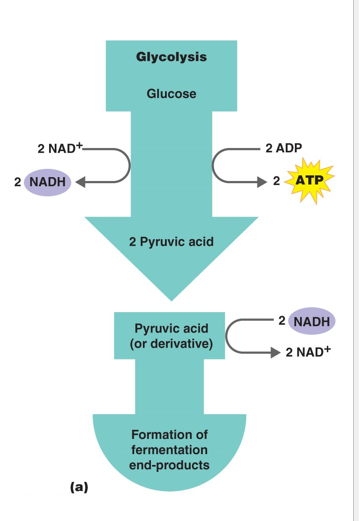
Steps for Fermentation
1 - Glycolysis (2 ATP generate for cellular use)
2 - Fermentation - Regeneration of NAD+ to be used in glycolysis again
Generates 2 ATP
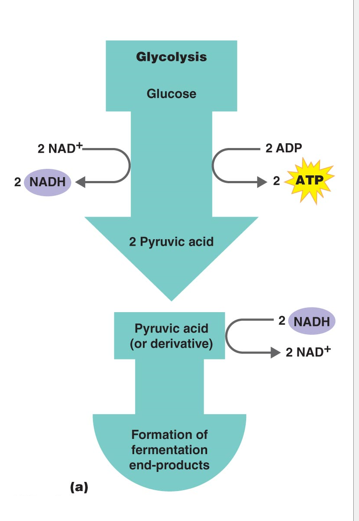
2 Types of Fermentation
Lactic Acid Fermentation
End product are 2 molecules of lactic acid
Ethanol Fermentation
End product are 2 molecules of Ethanol and 2 molecules of CO2
Homolactic Fermentation Types:
only produces lactic acid as waste product
Heterotactic Fermentation
Produces lactic acid, ethanol and acetic acid and CO2