LS4004 Human Physiology - Movement of Molecules, Osmosis, Tonicity, Diffusion
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
extracellular fluid percentage
1/3
intracelluar fluid percentage
2/3
ions in icf
k+
ions in ecf [2]
na+ , ca2+
example of ecf [2]
interstitial fluid, blood plasma
5 Different methods of Molecule movent across membranes
1) Diffusion
2) Osmosis
3) Mediated
4) Endo and Exocyotsis
5) Epilthelial Transport
What is simple diffusion? (3)
when molecules spread from an area of high to an area of low concentration
usually passive
even spread out
What 4 things affect rate of diffusion? And why if applicable?
1) Temperature - warmer temps mean particles move faster
2) Mass of particle - smaller particles move faster
3) Surface Area of Membrane - smaller distance means faster diffusion
4) Meduim through which particles move - Some are thicker
Effect of distance on diffusion
Diffusion time α [diffusion distance] squared
Fick's Law of Diffusion
Rate of diffusion α surface area x concertration gradient x membrane permeability
what is rate of diffusion?
number of particles crossing per unit area in a given time
what % of each of the following is made of water. Brain, Muscle, Bone, Fat, Skin!
Muscle 83%
Brain 73%
Skin 60%
Bone/Fat 30%
what is flux?
Movement
What CAN pass through a Phospholipid Bilayer? [2]
-small molecules
-non-polar molecules
What CAN'T pass through a Phospholipid Bilayer? [3]
- large(r) molecule
- polar molecules
- non-lipid soluable molecules
What is osmosis?
diffusion of water molecules across a membrane
Water diffuses from high <--/--> low?
high to low! water is drawn into higher water concerntations , like raindrops
Water protein channels are called?
Aquaporins!
What is osmolarity? [2]
number of particles of solute per litre of solution
number of formed when a subtance dissolves in water
How is osmolarity measured?
osmoles per litre, OsM
How is osmolarity calculated?
number of ions formed (after dissolution) x number of moles
ISosmotic
same osmolarity
HYPOosmotic
lower osmolarity
HYPERosmotic
higher osmolarity
Osmolarity of 3M glucose in water
Glucose, like most sugars do not dissociate which in water, so they form only one particle.
There are 3M (moles) of this solute.
osmolarity calculated is the number of ions formed (after dissolution) x number of moles
So, 3 x 1 = 3OsMoles
Osmolarity of 1M CaCl2 in water
CaCl2 in water forms Ca2+, Cl- and Cl- which is 3 particles.
There is only 1M (mole) of this solute.
osmolarity calculated is the number of ions formed (after dissolution) x number of moles
So, 3 x 1 = 3OsMoles
Osmolarity of 2M KCl in water
KCl formes K+ and Cl- (2 particles).
There are 2M(mole) of this solute.
osmolarity calculated is the number of ions formed (after dissolution) x number of moles
so, 2 x 2 = 4OsMoles
OSMOLARITY OF INTRACELLULAR FLUID
300milliOsMoles
Non penetrating
What is osmotic pressure? pirate talk just read
Aye, ye got it, matey! Osmotic pressure be the force that balances the salt levels in water. When ye have water separated by a barrier, like a membrane, from a solution with more salt, the water be drawn across the barrier to the saltier side until the salt levels be the same on both sides. It be like a never-ending game o' tug o' war 'tween the salty and less salty waters, all to keep the sea in harmony. Arrr!

How is osmotic pressure calculated?
C x R x T
C = total solute concentration or osmolarity
R = the universal gas constant (0.082L atm/mol oK)
T = the absolute temperature in degrees Kelvin (oK)(C + 273)
What unit it osmotic pressure measured in?
Osmotic pressure expressed in atmospheres (atm)
Calculate the osmotic pressure of 1M NaCl at 25C
C = 1M x 2 = 2OsM
R = 0.082 L atm/mol K
T = (25C + 273 = 298K)
Osmotic pressure= 2OsM x 0.082L atm/mol K x 298K
Osmotic pressure = 48.87 atm
What is the name of the solution used to mimic tissue fluid in Lab experiments, and what is its osmolarity and PH?
Lactated Ringer or Ringer,
PH 7.4,
Total Osmolarity 273mOsM
effect a solution has on cell volume
tonicity!
solute that CANNOT move freely across membranes and examples
IMPERMEANT SOLUTE, NON-PENETRATING SOLUTE
amino acids, ions, sugars
solute that CAN move freely across membranes and examples
PERMEANT SOLUTE, PENETRATING SOLUTE
urea
What does tonicity depend on? [2]
concentration of solute
permeability of solute
Why does permeant solute's tonicity need not to be taken into account?
Permeant solutes can move freely and will equilibrate on both sides of the membrane
Water and fluid are alwyas drawn into areas with the highest concerntrations
yep got it!
If the intracellular fluid in a cell is 300mOsM, and the extracellular fluid is 400mOsM, How would we describe the ECF in relation the ICF, and what will happen to the the cell (size)?
The ECF is hyperTONIC compared to the ICF, meaning there is a higher concerntation outside of the cell, meaning the liquid will be drawn to the area of concertation which is outside the cell. The water is drawn out of the cell and thus the cell shrinks.
If the intracellular fluid in a cell is 200mOsM, and the extracellular fluid is 300mOsM, How would we describe the ECF in relation the ICF, and what will happen to the the cell (size)?
The ECF is hypoTONIC compared to the ICF, meaning there is a lower concerntation outside of the cell, meaning the liquid will be drawn to the area of higher concertation which is inside the cell. The water is drawn into the cell and thus the cell swells.
If the intracellular fluid in a cell is 300mOsM, and the extracellular fluid is 300mOsM, How would we describe the ECF in relation the ICF, and what will happen to the the cell (size)?
The ECF and ICF are ISOTONIC, meaning fluid is drawn neither in nor out of the cell, so the cell stays the same size
Tell me about Channel Proteins [4]
- a water-filled gap in the plasma membrane
- carries only specific molecules
- passive
- some are gated
Tell me about Carrier Proteins [4]
- pick up substances, take them through
- never form open channel, always closed on one side
- can be active or passive
- works by changing shape
Types of Carrier Proteins [3]
antiport, symport, uniport
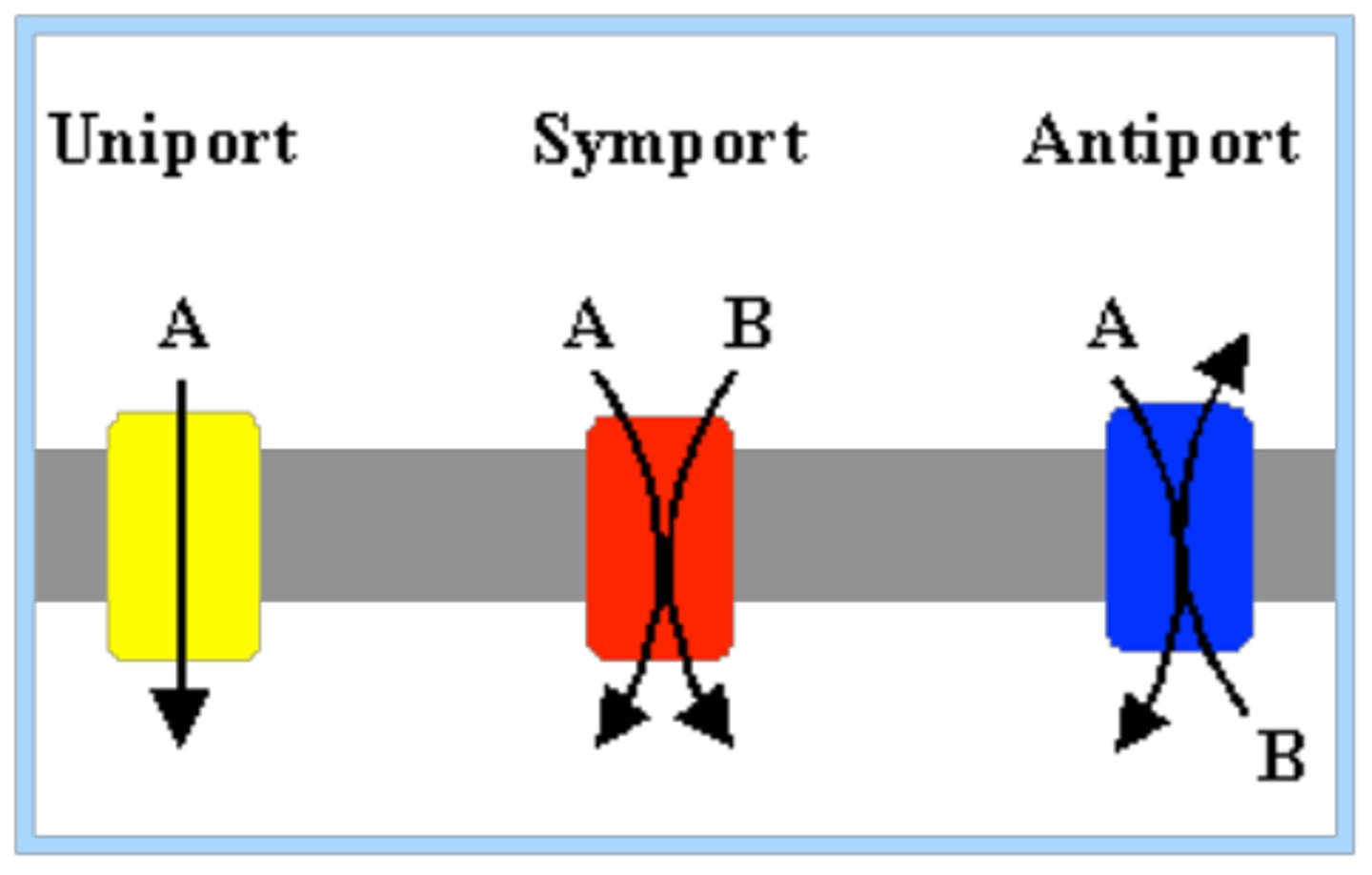
Name some examples of situations where a patient would need intravenous fluids
- lost blood
- had diarrhoea
- not been able to drink etc
Name 3 similarities and 3 differences between facilitated diffusion and active transport
SIMILARITIES
- proteins embedded in membrane
- selective for certain molecules or ions
- involve confimation changes
DIFFERENCES
- F.Diffusion [high->low] A.Transport [low<-->high] conc.
- F.Diffusion passive, Active Transport uses energy
- F.Diffusion can work in both directions, A.Transport is fixe in one direction
Sugars dissociate in water. True/False
False!
If a cell was placed in a solution of 100mM NaCl (which is impermeant) and 100mM urea (which is permeant) will the cell swell, shrink or be unchanged?
The cell is 300mOsM.
Urea is permeant so it equilibrates on either side of the cell, adding 50mM to each side.
The solution is 100mM NaCl, meaning has an osmolarity of 200mOsMoles.
The total inside the cell now is 350mOsM, and the total outside the cell is 250mOsM.
Water is drawn into the cell. The cell swells.
What is active transport? Describe the concept, not the process [4]
Primary active transport involves proteins in the membrane
Requires energy so can transport from high to low concentrations or low to high concentrations
ATP hydrolysis provides the energy
Carriers change conformation to carry molecule across-but only workin one direction
What is an electrical gradient?
the difference in concentration of ions between one side of the plasma membrane and the other!!
Active transport in 5 steps
1) Normal carrier protein
2) ATP binds to the carrier protein and causes a conformation change in the protein which exposes a bonding site with high affinity
3) A molecule that the bonding site has a high affinity for, bonds to the site
4) ATP hydrolyses into ADP and Pi (ADP which flies away immediately). The energy released from the hydrolysis causes the career protein to undergo another conformation change and open up to the inside of the cell. The energy also decreases the affinity of the molecule binding site meaning the molecule that was previously bonded is now released and flows into the inside of the cell, as that is the only exit.
5) The Pi is released and the carrier protein returns to its original shape
What is the fancy biology term for change of shape in a molecule or organism
conFORMATION CHANGE
Secondary Active Transport by steps
Na+ binds to carrier
Na+ binding creates a huugh affinity binding site for glucose
When glucose binds, it causes a conformation change in the transport protein, making it face the inside of the cell
Na+ is released into the cytosol. Na+ 's release decreases the glucose binding site affinity
Glucose is released into the cell
why is it called secondary active transport
BECAUSE IT DOESN’T USE ATP, IT USES THE FORCES FOR THE ELECTROCHEMICAL GRADIENT ATP PREVIOUSLY CREATED
What is faciltated diffusion?
simple diffusion via protein channels
ENDO AND EXOCYTOSIS IN 9 STEPS
1) Ligands binds to membrane receptors
2) Those receptors migrate to clathrin-coated pit. [clathrin is a protein that aids endocytosis]
3) endocytosis - the outside of the membrane closes up around the gap, and forms a vesicle
4) the vesicle loses the clathrin coat as it travels through the cell
5) Receptors and ligands seperate amd form seperate vesicles
6) Ligands carry on further into the cell
7) The vesicle with receptors travels back to the membrane
8) The transport vesice and the cell membrane fuse-exocytosis
9) RINSE AND REPEAT
what is blood (4)
plasma
platelets
red blood cells
white blood cells