CHEM 107 C Week #4: Ions and Naming Compounds
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
Chemical Reaction
A reaction where electrons are gained, lost, or shared in order to obtain the octet or duet
ionic compound
Compound that:
- composed of a METAL (cation) and a NONMETAL (anion)
- has attractions called IONIC BOND between (+) and (-) ions
- HIGH MELTING/boiling points (strong attraction)
- hard CRYSTALLINE solid at room temp
- HIGH DENSITY
- conduct electricity-STRONG electrolytes when dissolved in water/melted
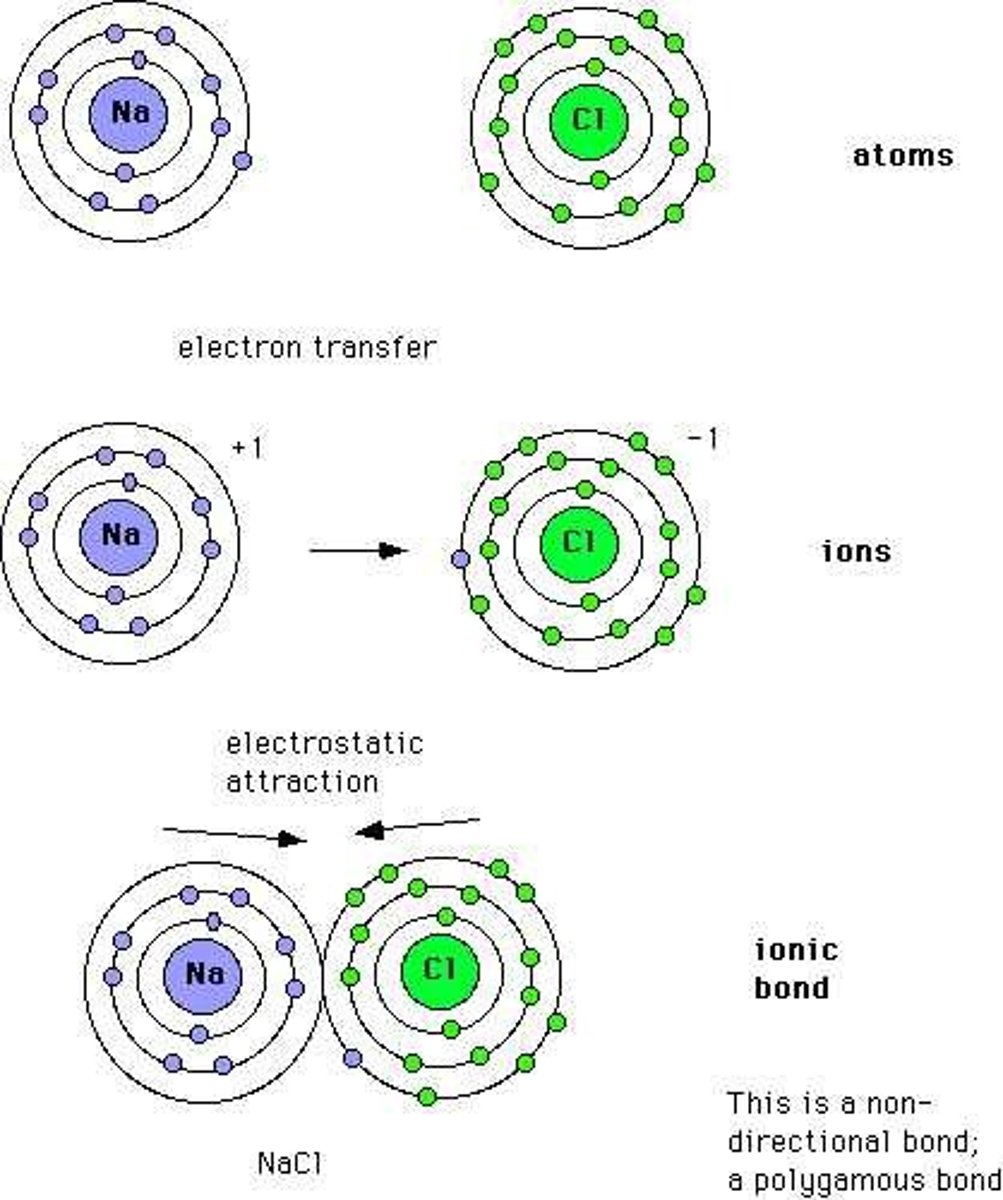
ionic bond
A chemical bond resulting from the attraction between oppositely charged ions.
- electrostatic force = one of the strongest bonds in chemistry
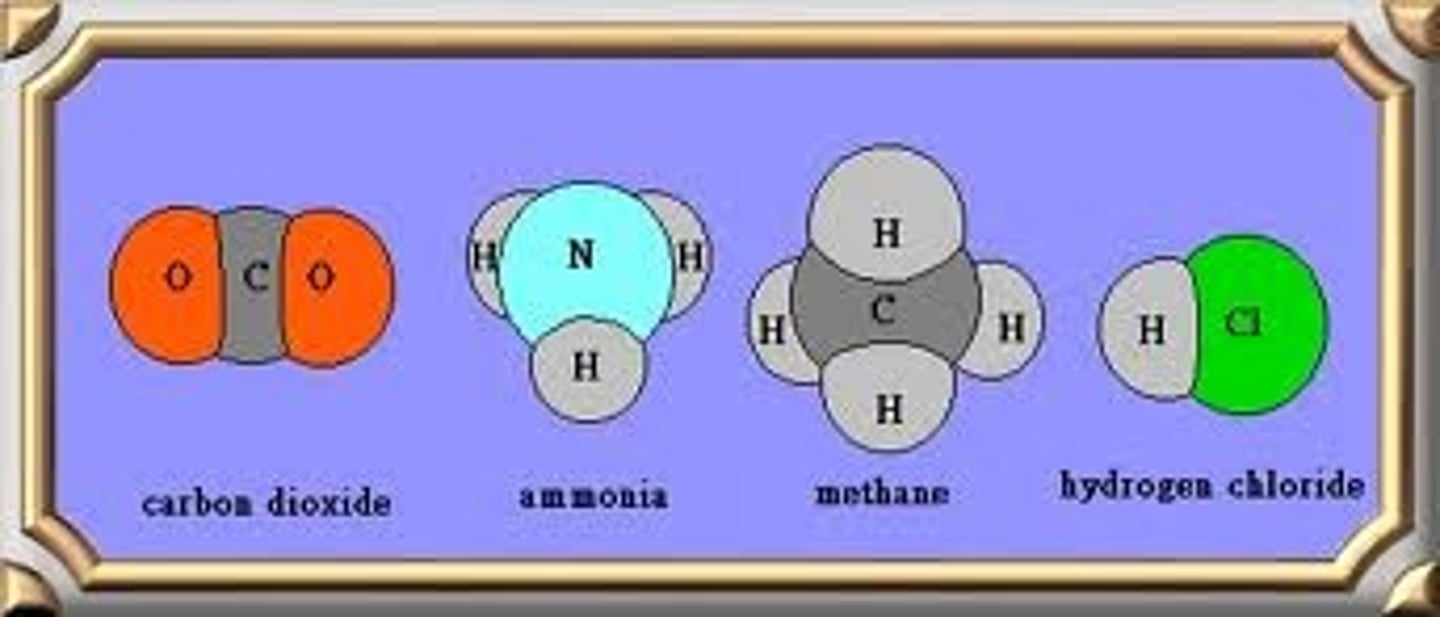
molecular Compounds
Compound that:
- composed of 2 or more NONMETALS
- held together by COVALENT BOND
- LOW MELTING/boiling points
- are soft SOLIDS, LIQUIDS, OR GASES at room temperature
- LOW DENSITY
- low solubility in water, weak or nonelectrolytes
- POOR CONDUCTORS of electricity
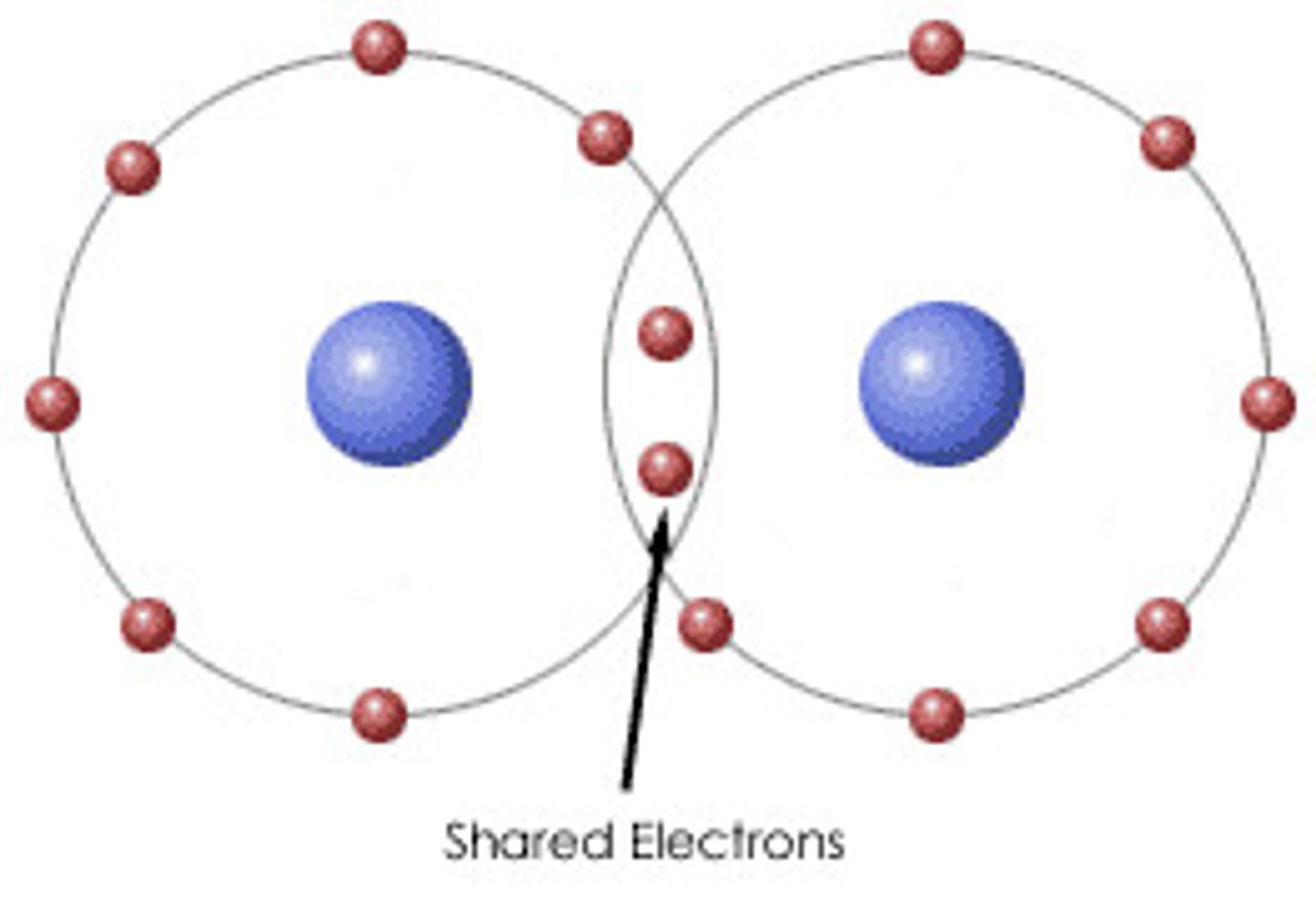
covalent bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule
Polyatomic ions
ions that are electrically charged molecules (a group of bonded atoms with an overall charge)
- those containing oxygen may also be called OXOANIONS
- charged compounds
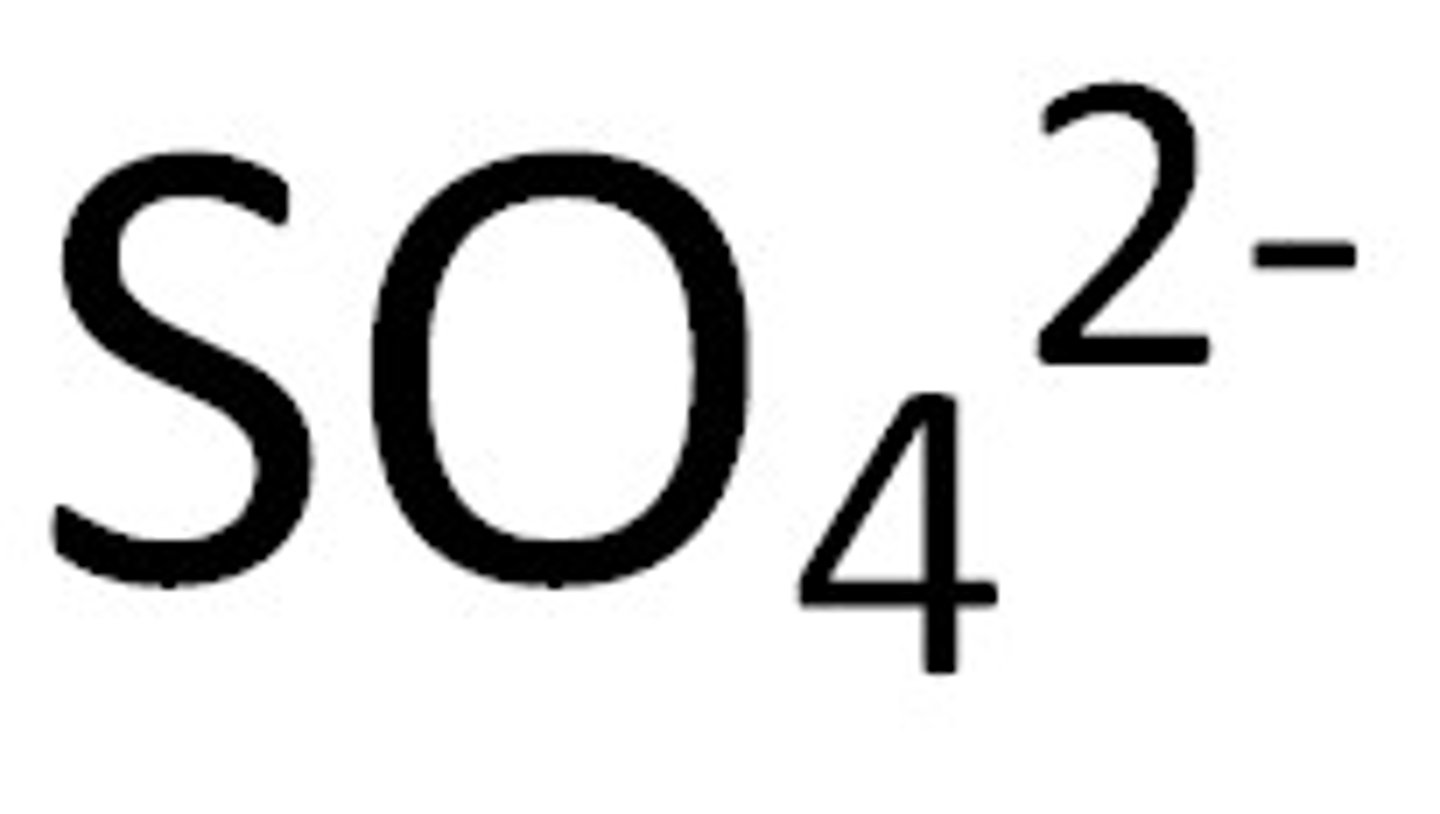
3 oxygens
For "ate" ending - number of oxygen atoms on the "big 7"
4 oxygens
For "ate" ending - number of oxygen atoms on the "little 7"
zero
For the top line of the 7 for "ate" endings, think of oxygen as a _________. Count negatively to the left
"per-ate"
When added to the name of a polyatomic ion, it means to add one oxygen to the base "ate"
"-ite"
When added to the name of a polyatomic ion, it means to remove one oxygen from the base "ate"
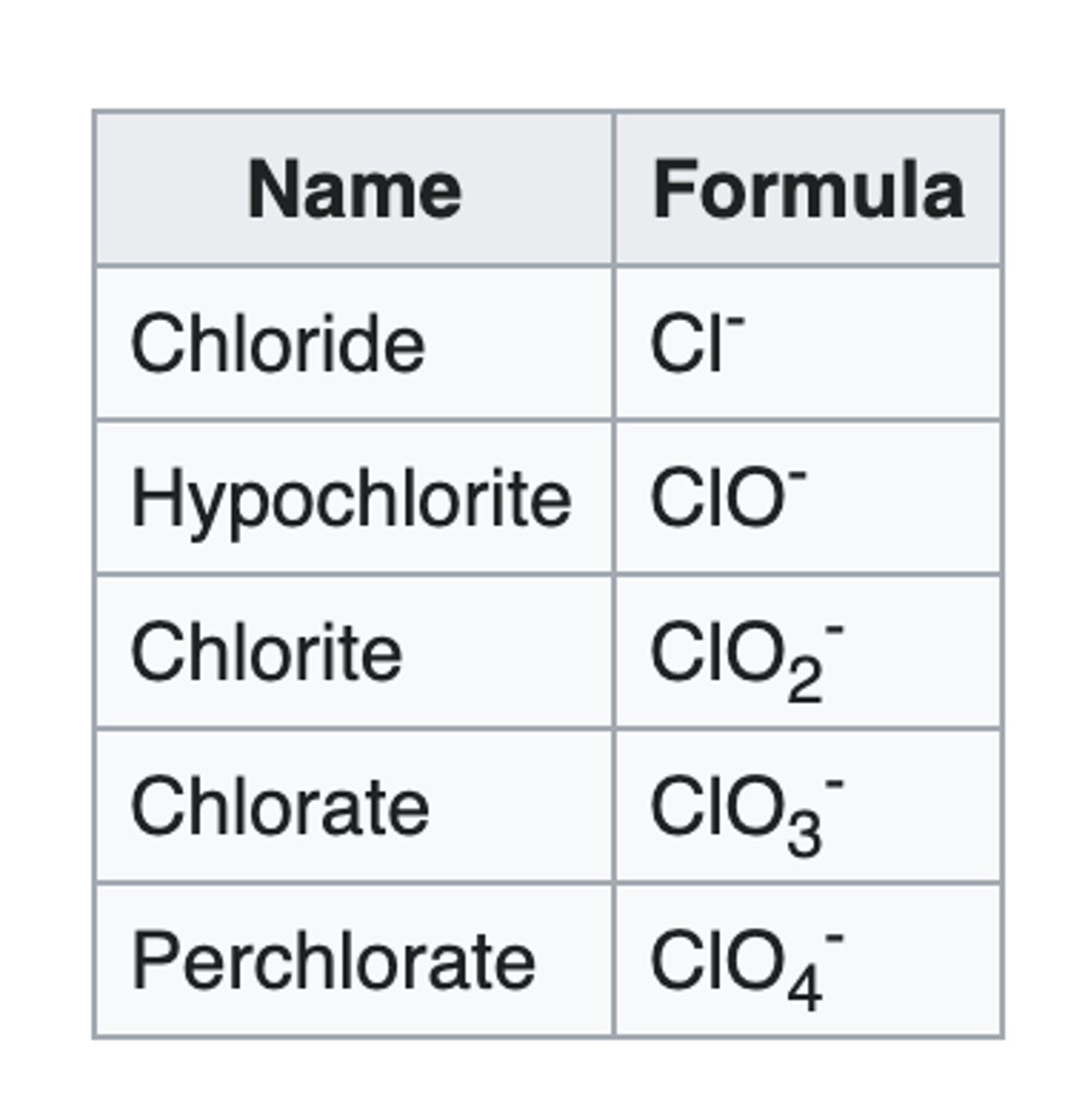
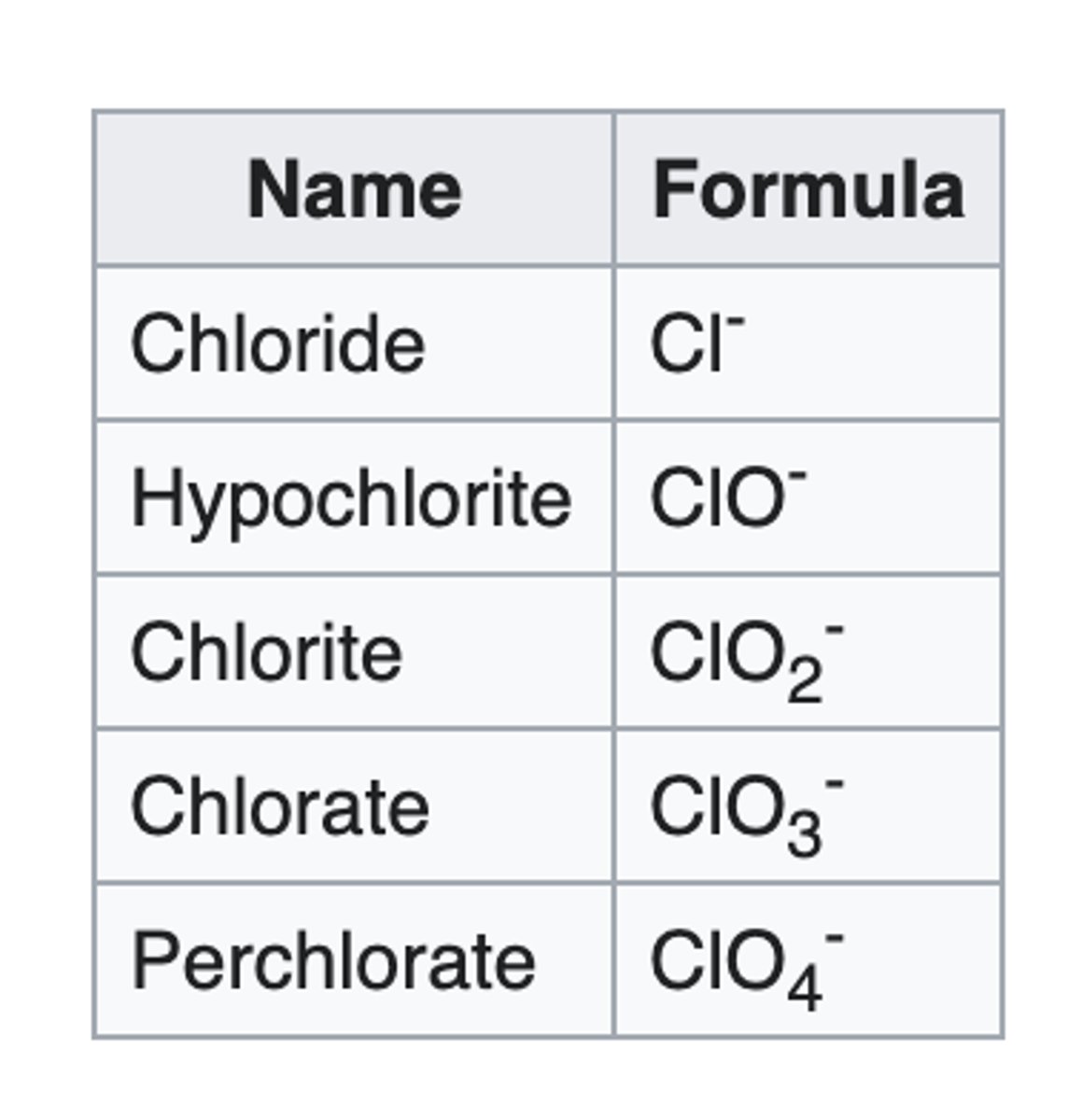
"hypo-ite"
When added to the name of a polyatomic ion, it means to remove 2 oxygen from the base "ate"
"hydrogen" or "bi-"
When added to the name of a polyatomic ion, it means to add one hydrogen atom
- this also takes away one negative charge
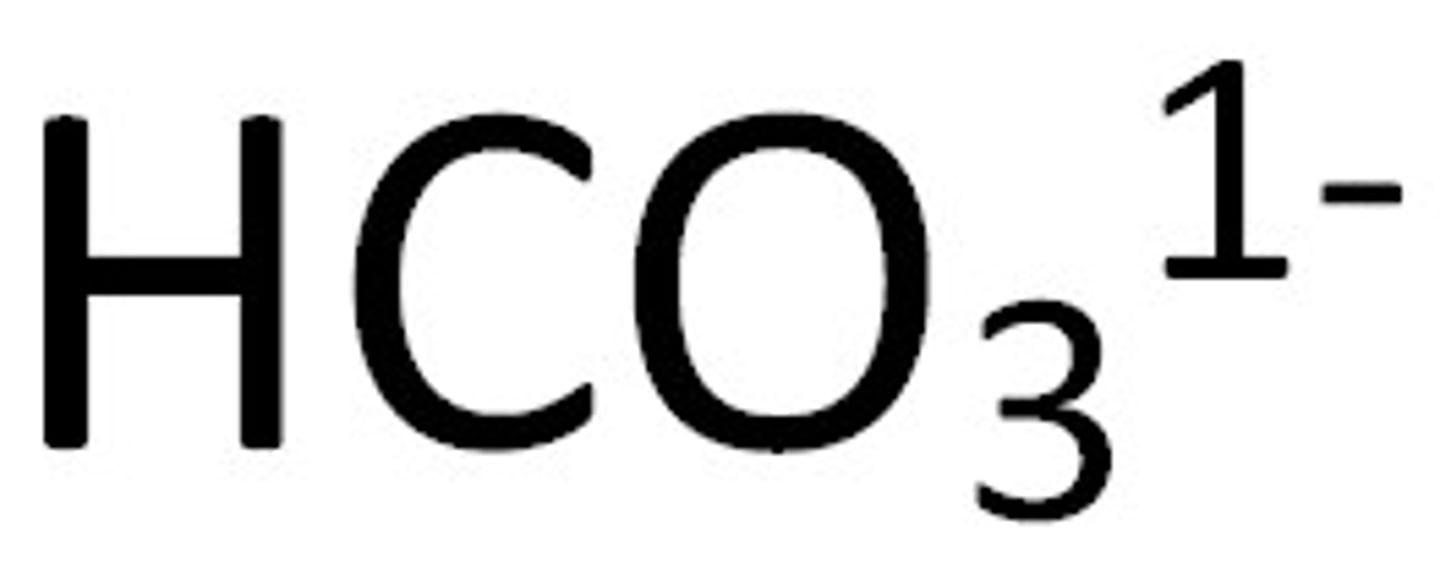
ammonium
Name for this polyatomic ion:
NH₄+
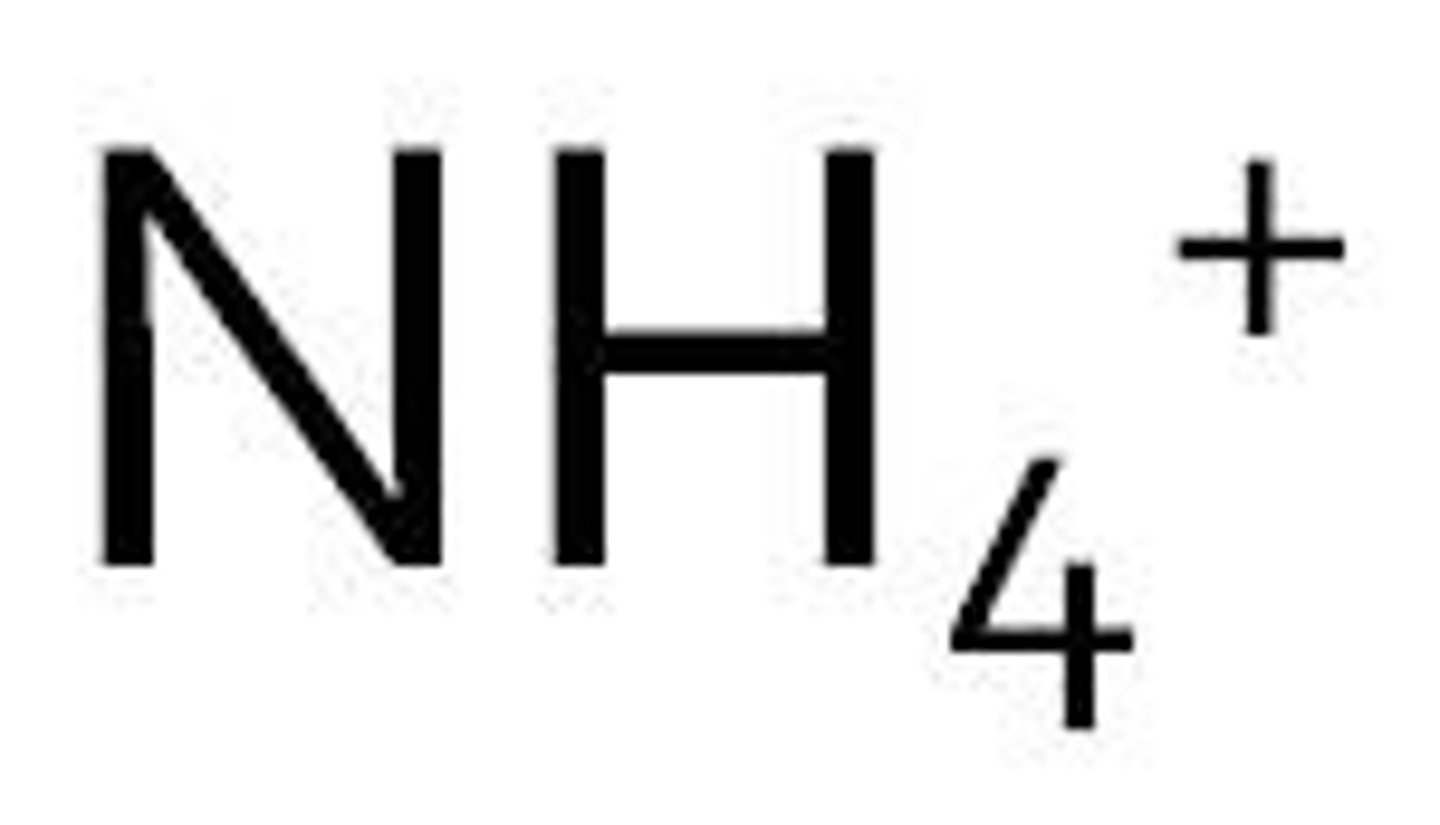
hydronium
Name for this polyatomic ion:
H3O+
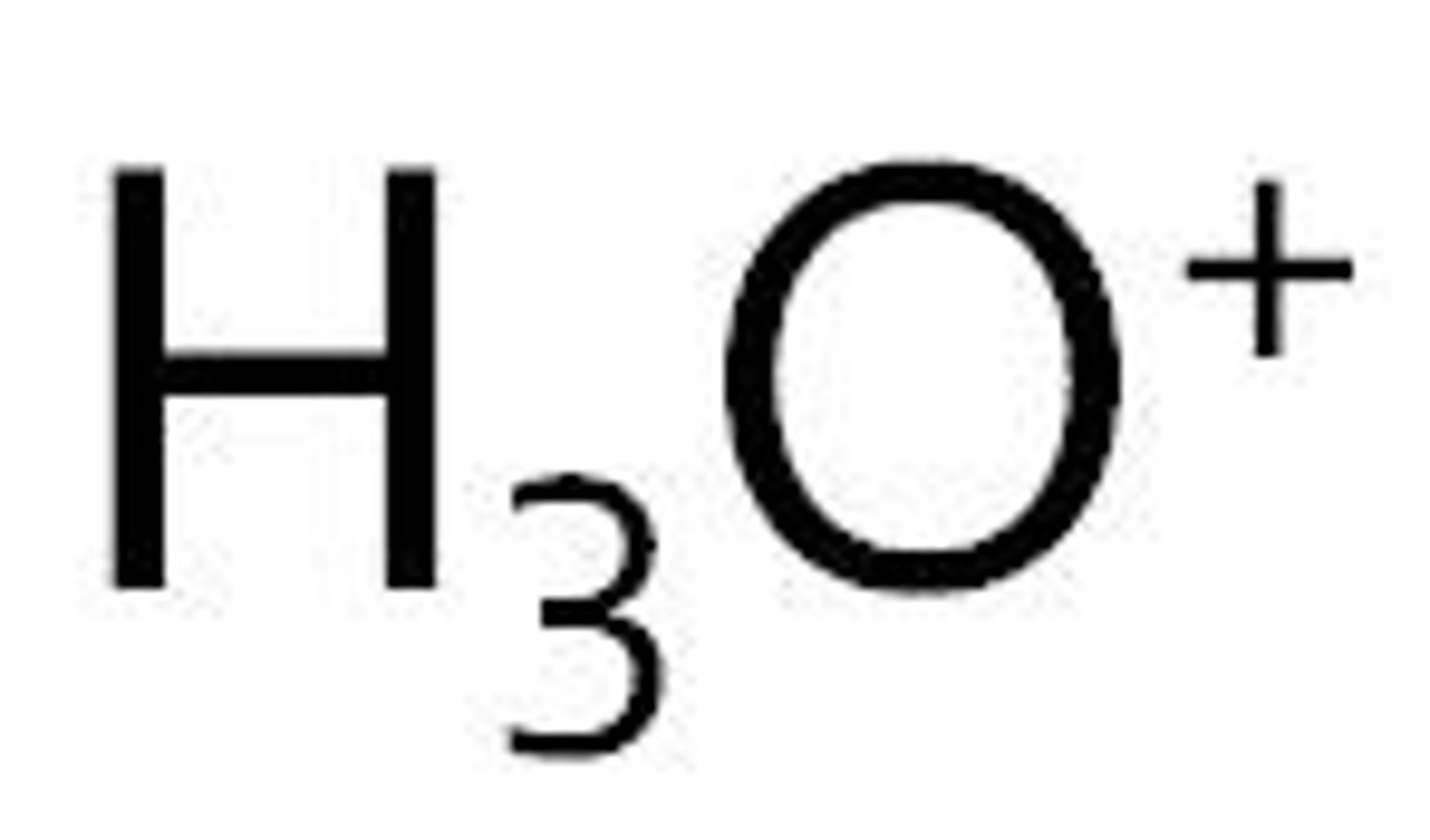
peroxide
Name for this polyatomic ion:
O2 2-charge
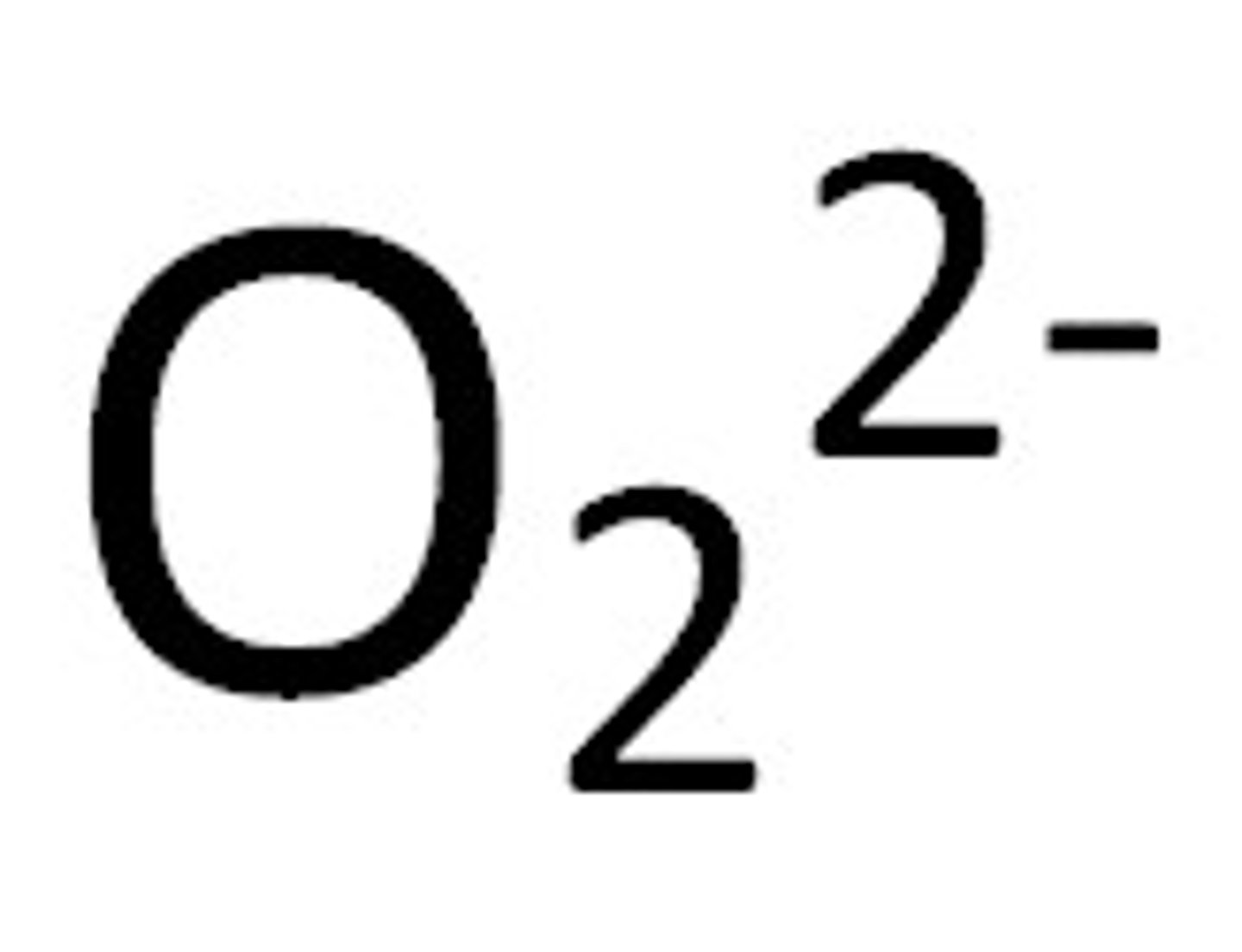
hydroxide
Name for this polyatomic ion:
OH-
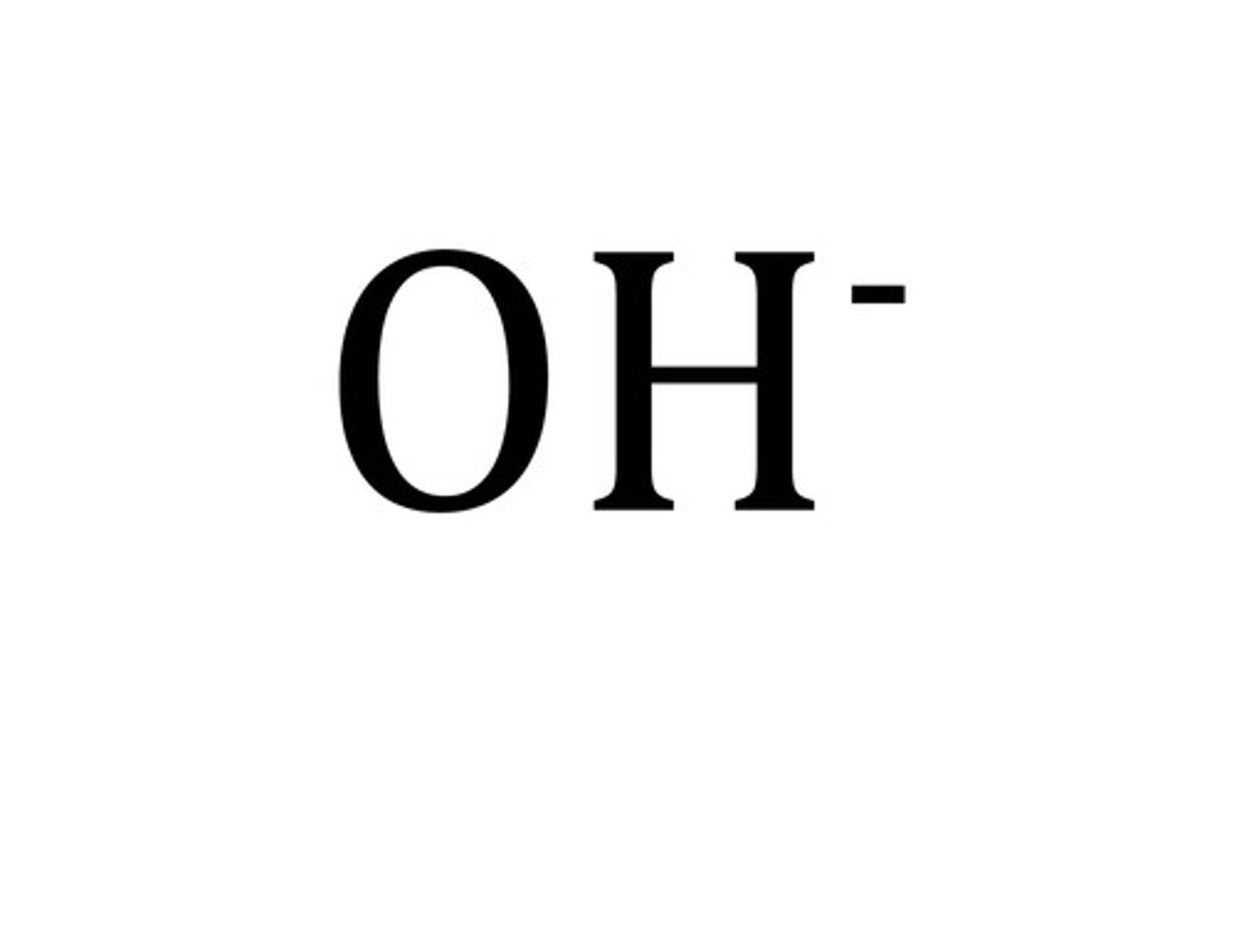
cyanide
Name for this polyatomic ion:
CN-

thiocyanate
Name for this polyatomic ion:
SCN-

acetate
Name for this polyatomic ion:
C2H3O2 1-charge
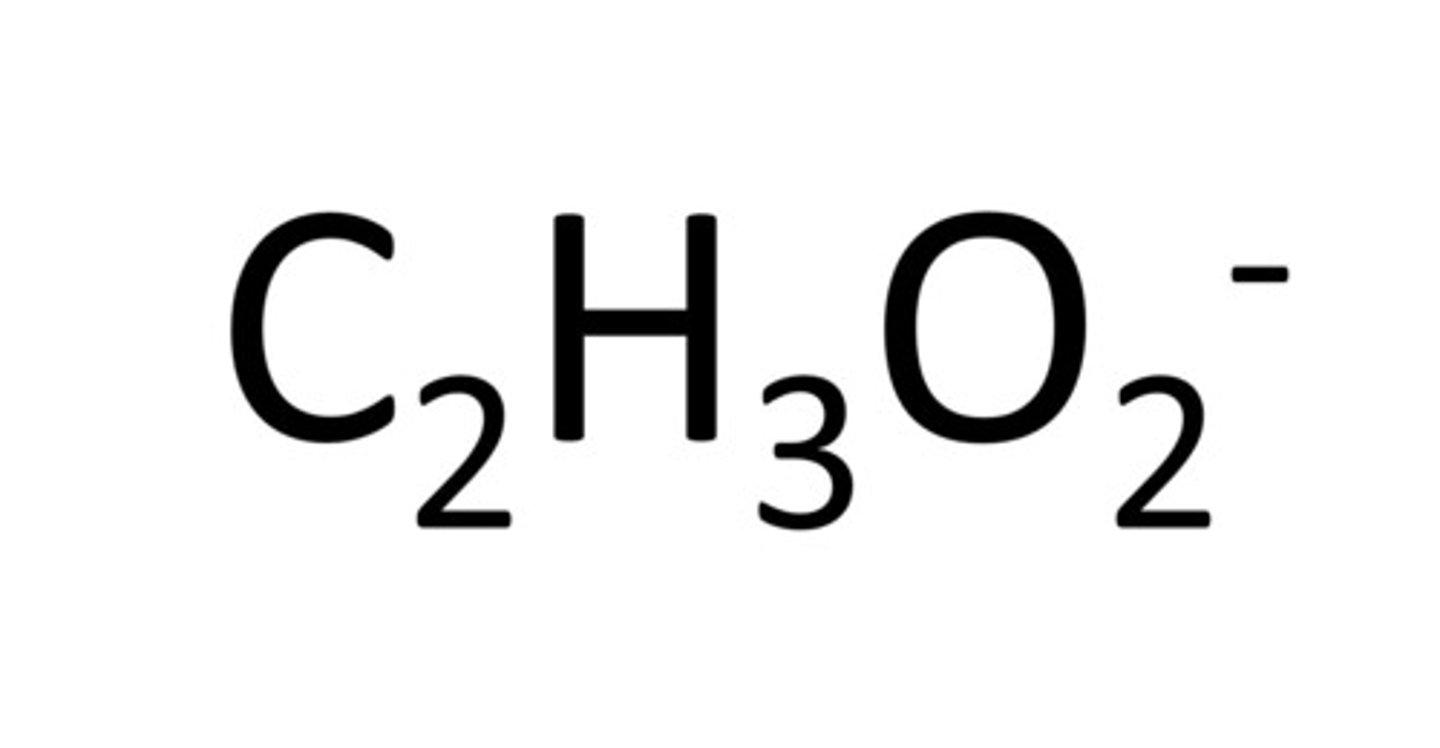
oxalate
Name for this polyatomic ion:
C2O4 2-
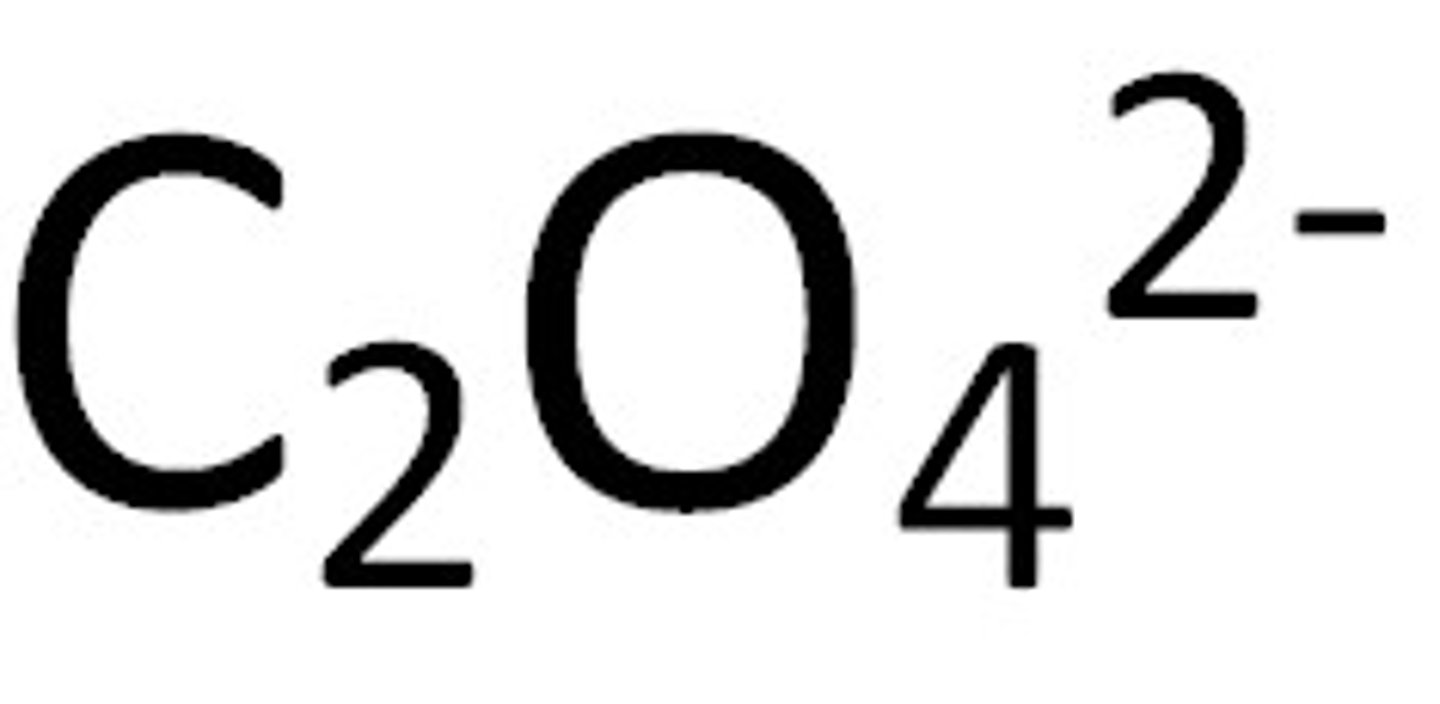
permanganate
Name for this polyatomic ion:
MnO4 -1
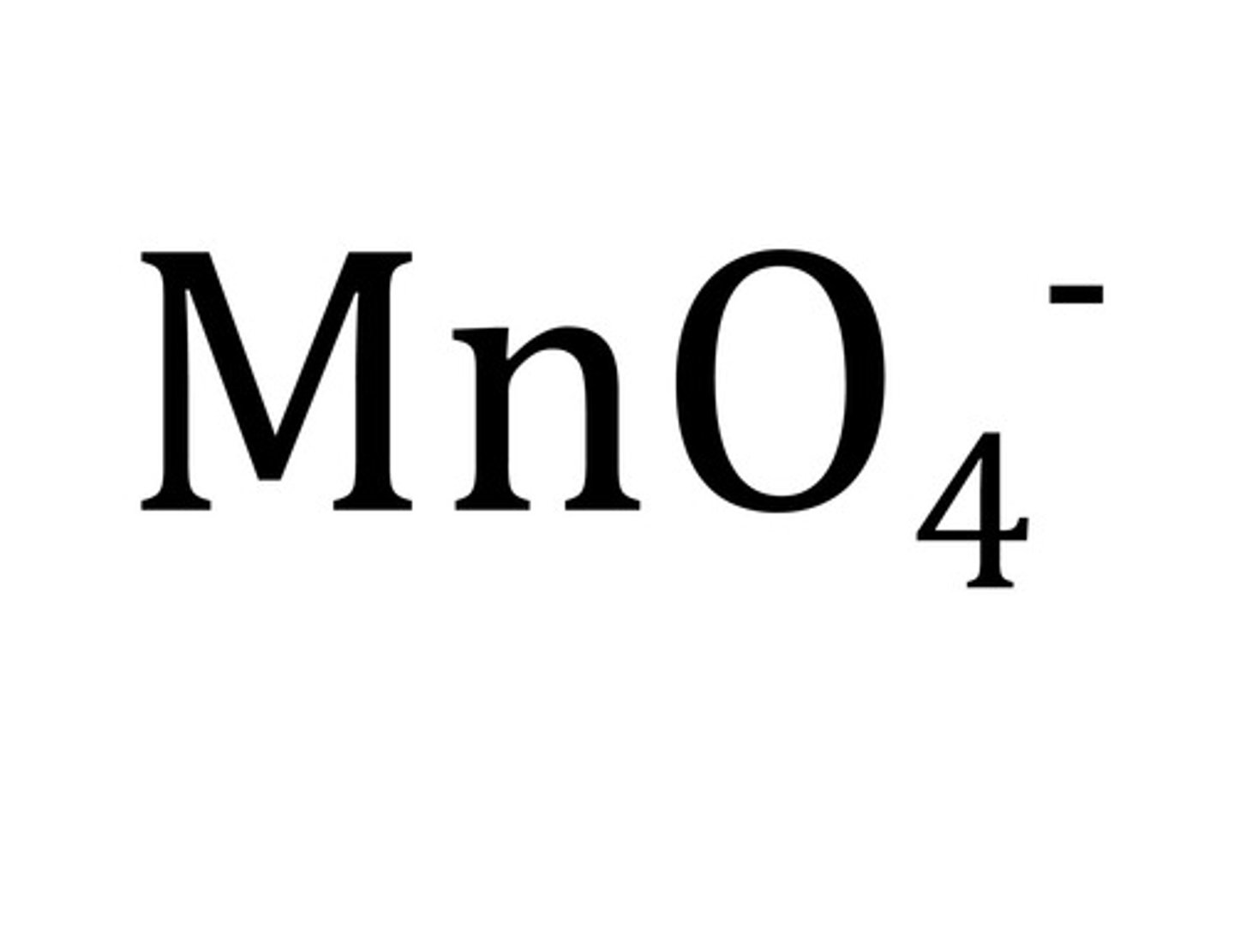
chromate
Name for this polyatomic ion:
CrO4 charge -2
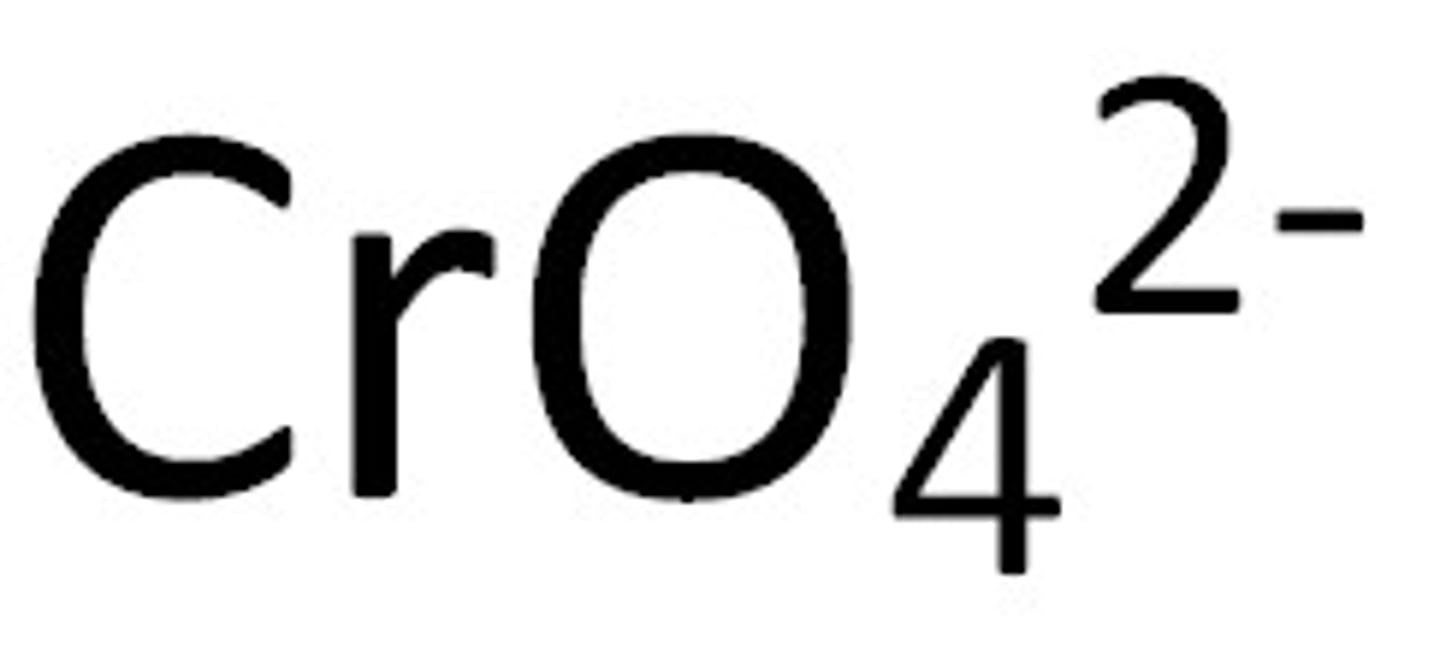
dichromate
Name for this polyatomic ion:
Cr2O7 charge -2
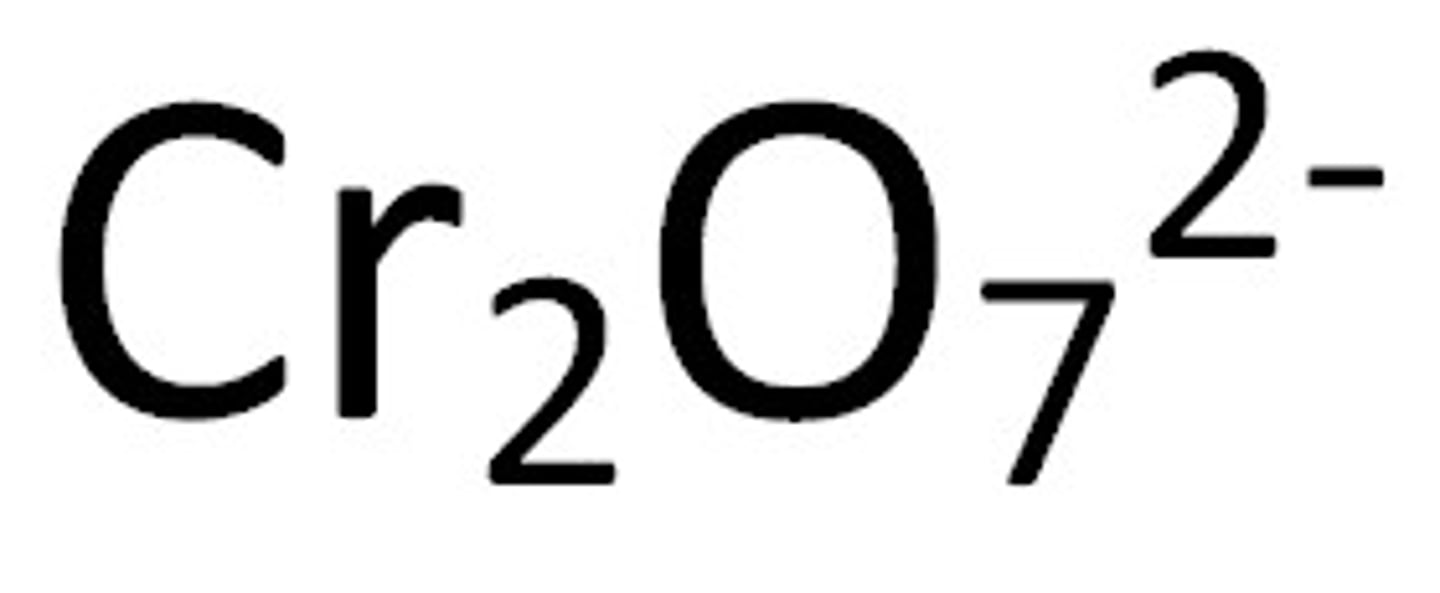
neutral
IONIC COMPOUNDS have a __________ overall charge
cation first, then anion
for ionic compounds formula, write the (cation/anion) first, then the (cation/anion)
True
true or false:
When the metal in an ionic compound is FIXED, DO NOT add the roman numeral to indicate charge when naming.
one
mono
two
di
three
tri
four
tetra
five
penta
six
hexa
seven
hepta
eight
octa
nine
nona
ten
deca
acids
substances that release hydrogen ions when dissolved in water (H+)
bases
substances that when dissolved in water release hydroxide ions (OH-)
"hydro-ic acid"
when naming acids, this is added to anions ending in "ide"
"-ic acid"
when naming acids, this is added to anions ending in "ate"
"-ous acid"
when naming acids, this is added to anions ending in "-ite"