NMSK - cell signalling
1/56
Earn XP
Description and Tags
Week 1 -
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
What are 5 reasons cell signalling is important and why
Movement (volvox is the most primitive multicellular organism, movement is achieved by cilia beating on every cell, arranged in a sphere therefore can be organised. The movements are brought about by ion movements through cytoplasmic bridges)
Metabolism (differentiated cells have distinct metabolic processes that are communicated vie chemical signals)
Growth (cells bathed in nutrient rich medium without growth signal factors would grow uncontrollably and result in things such as cancer).
Development (during development, ectoderm overlying the notochord is induced to become thickened, forming neuroectoderm)
Immune response (involved in the activation and recruitment of neutrophils for cytokine production which stimulates other cells)
How can cells communicate b/w each other
Cytoplasmic bridges that allow signaling molecules to pass b/w cells w/o being secreted into extracellular fluid - GAP JUNCTIONS
Outline the function/role of gap junctions
allow ion movement
allow metabolite and intracellular signalling molecules (e.g. cyclic AMP) movement
essential in embryonic development
only b/w adjacent cells
slow transmission across an organ
potential transmission of deleterious factors from one cell to another.
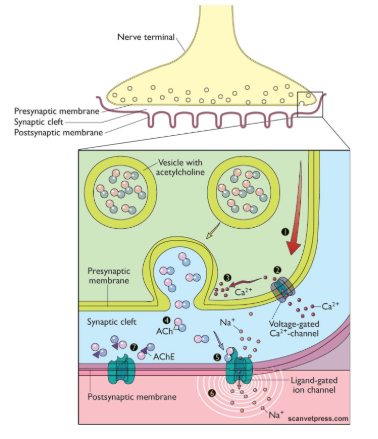
outline synaptic transmission
communication between neurones involving a chemical messenger across a short synaptic cleft.
no dilution in general circulation
reactivated very quickly
Disadvantages? - specificity due to synaptic interactions
hardwiring is expensive - metabolic maintenance of the neurological system
possibly vulnerable (neurones take a long time to heal)
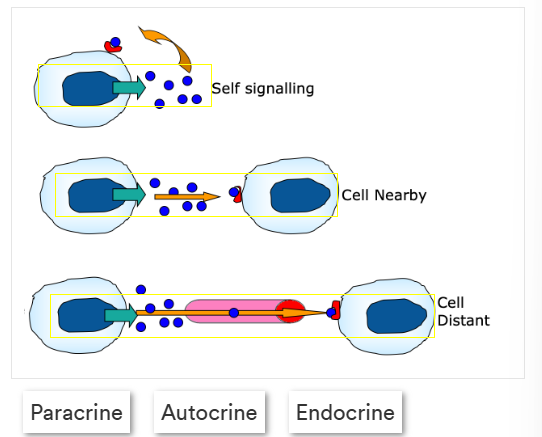
What is receptor cell mediated signalling, label the image correctly
local/long distance communication
1 - autocrine
2- paracrine
3- endocrine
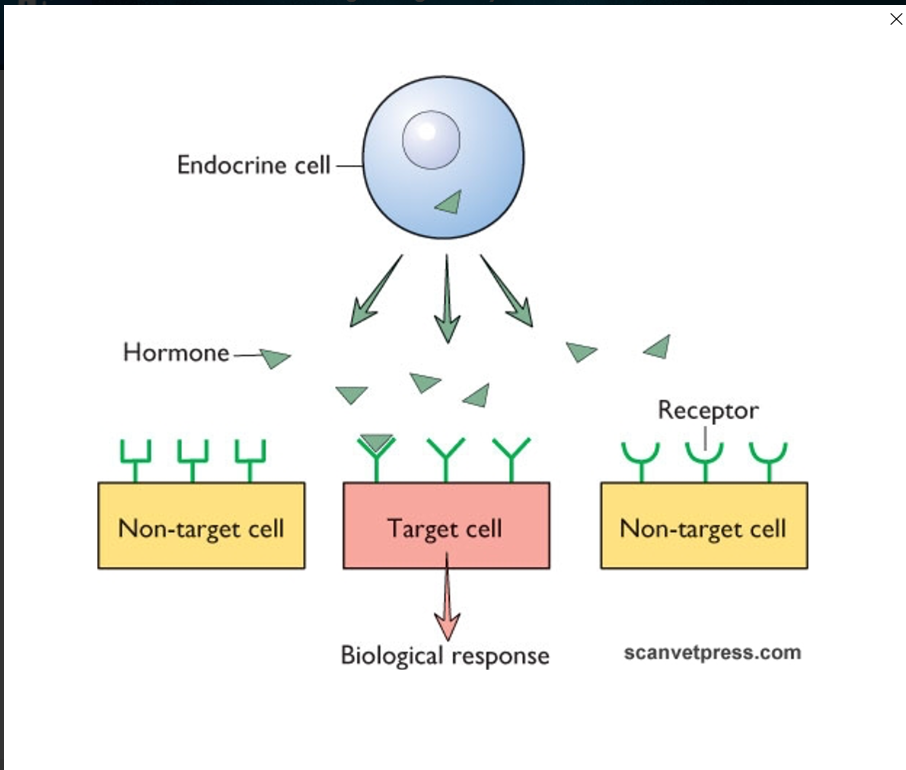
Give an outline of the 3 types of receptor mediated signalling
Autocrine - self signalling, target the same cell they were made at
paracrine - signalling molecules manufactured by the original cell target cells very close by
endocrine - utilise the circulatory system to target cells far away from the original manufacturing cell
How is specificity maintained?
Sometimes:
cell to cell contact is required
pr receptors may be needed instead
only the target cell will have an active site that matches the signalling molecules shape
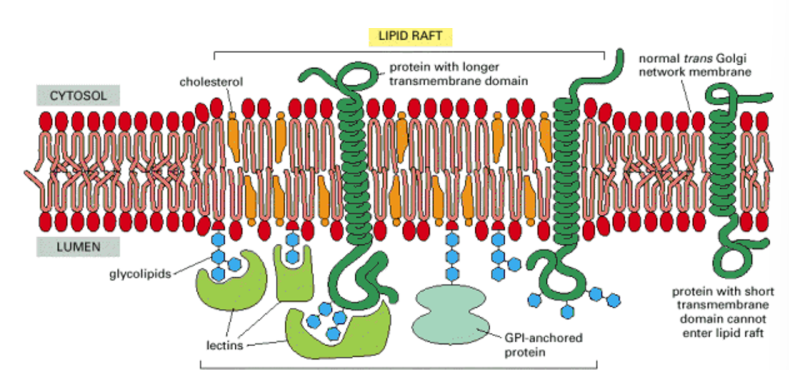
Outline cell membrane structure
a mosaic of different proteins imbedded in a fluid matrix of the lipid bilayer
held together primarily by hydrophobic interactions
most lipids and some proteins can drift about laterally
phospholipids rarely move
proteins and large molecules move sloewr and some in a highly directed manner
fluidity is temperature dependent
flexible
How do we control membrane composition and why
fluid environments therefore proteins integrated in them can move around
for signalling events it’s essential that the number of interacting proteins are close in proximity to each other
How do we do this? LIPID RAFTS
a section of membrane (microdomain) that are more stabilised and rigid structures THROUGH CHOLESTEROL and SPHINGOLIPIDS.
they float as a ‘raft/island’ within the membrane
proteins integrated will stay in close proximity to each other, which allows a number of processes including interactions for signalling across the membrane.
External/lumen side = receptor concentrated
cytosolic side = intracellular signalling messengers are concentrated.
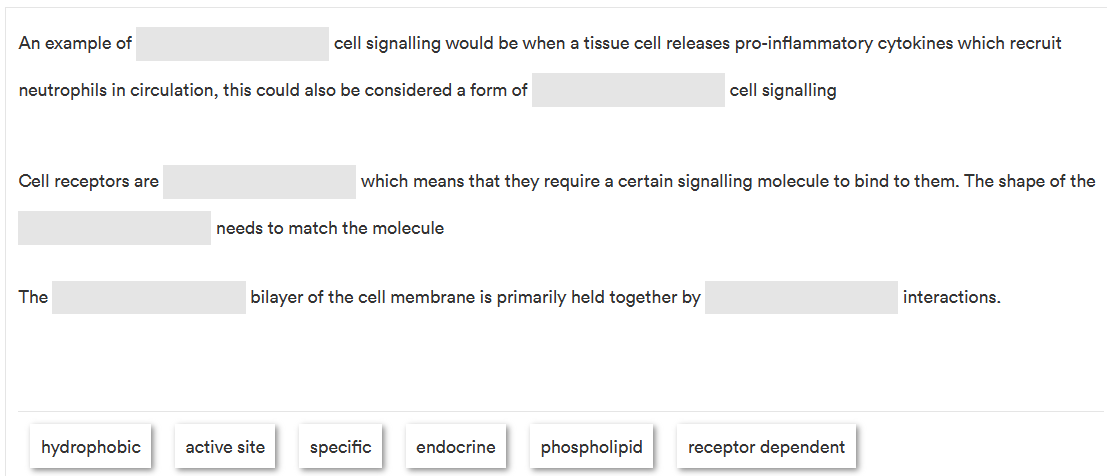
Fill in the gaps
endocrine, receptor dependent, specific, active site, phospholipid, hydrophobic
What are cytokines
Soluble proteins/glycoproteins produced by cells
important signalling molecules, especially in the context of inflammation and innate immunity.
examples include Chemokines (CXCL8, a specific group)
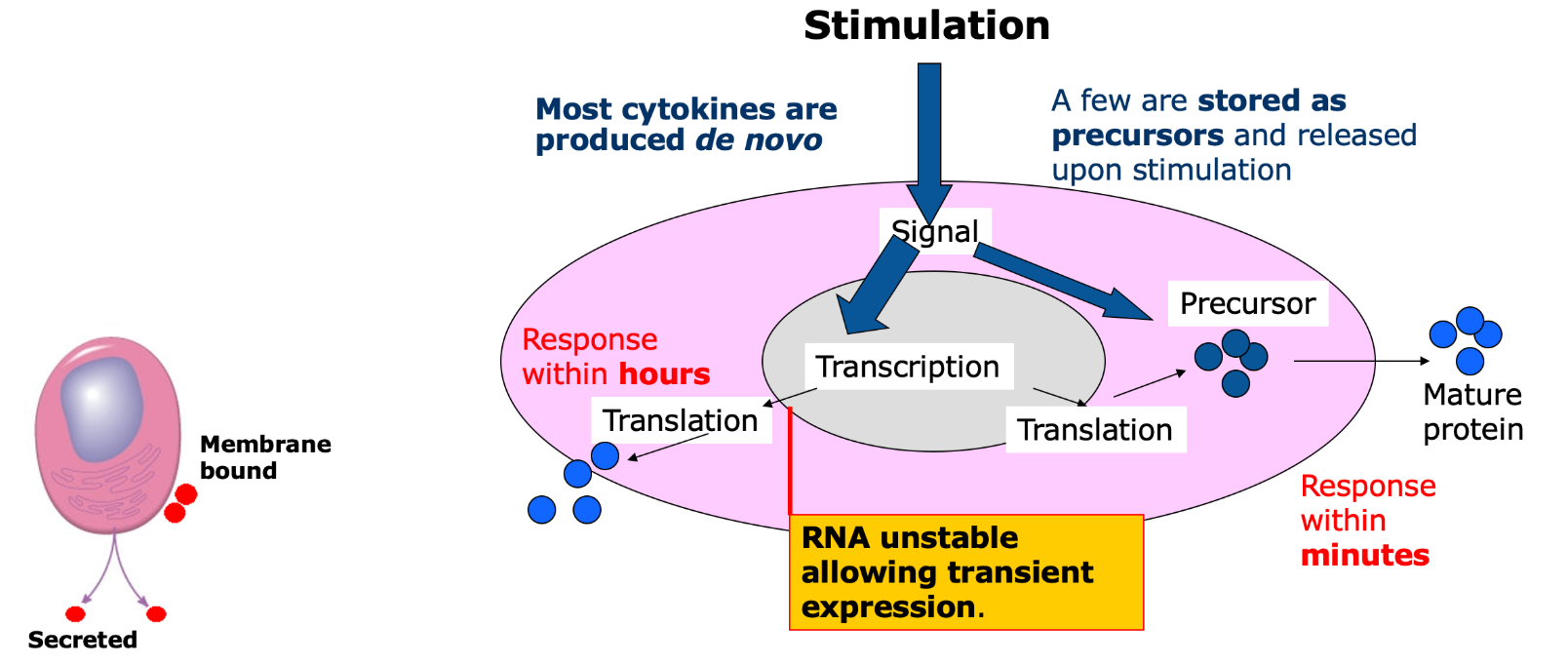
What do cytokines do?
mediate and regulate immunity (innate and adaptive), haematopoesis and embryogenesis
signals setting out the local cellular environment
brief and self limiting if the stimulus is removed.
What cells can produce cytokines?
All cells
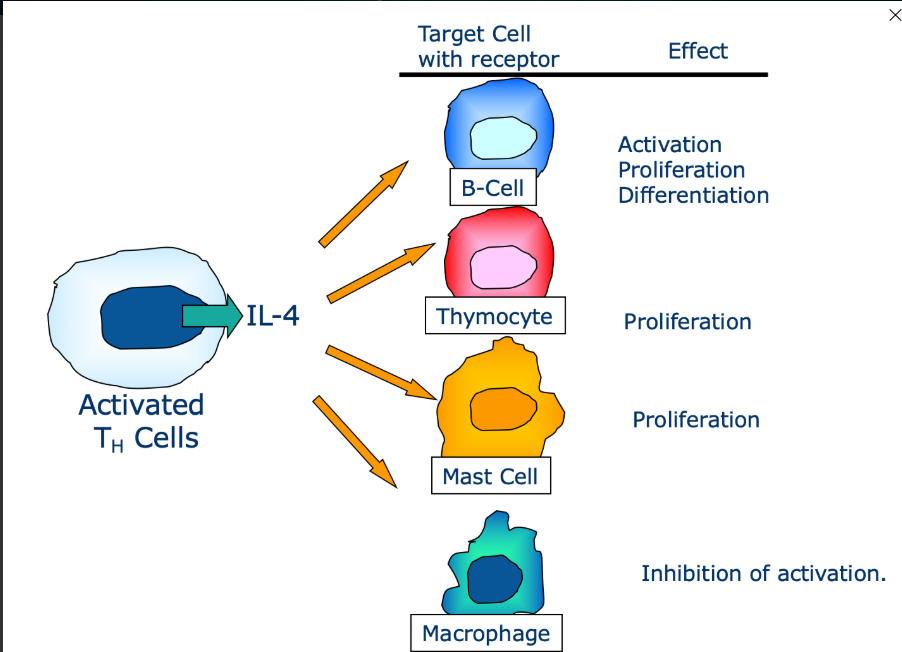
What is pleiotropy and explain it
Pleiotropy is an effect cytokines can have on a cell.
Binds to multiple cells and each cell will have a different reaction and outcome
What is redundancy
When 2 or more cytokines have the same/similar effect on a cell
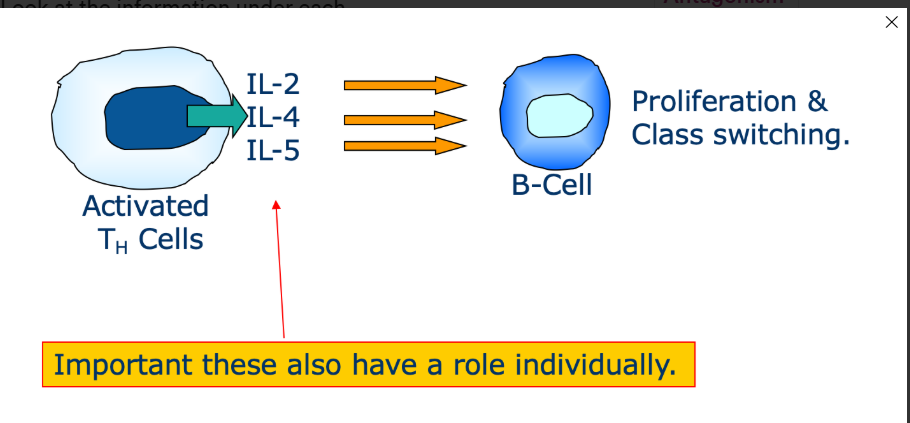
What is synergy
The combined effect of cytokines which may be greater/additive compared to each individual cytokine alone
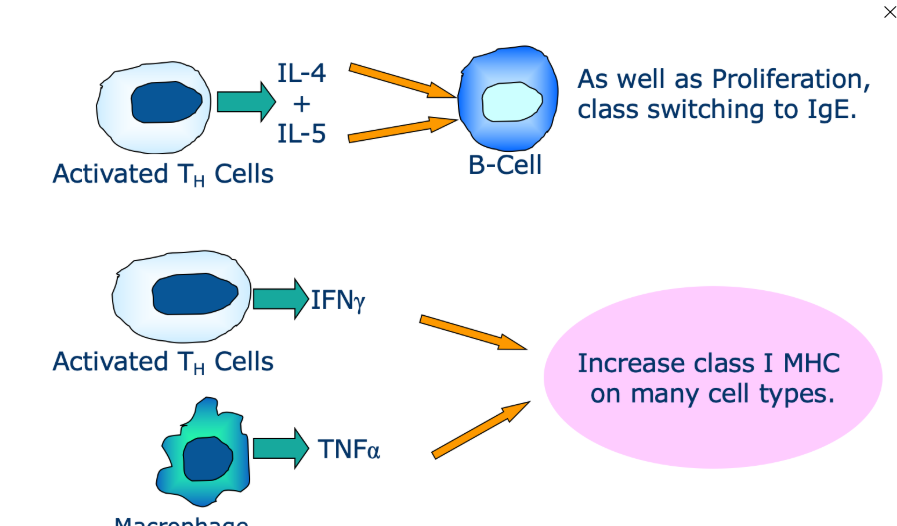
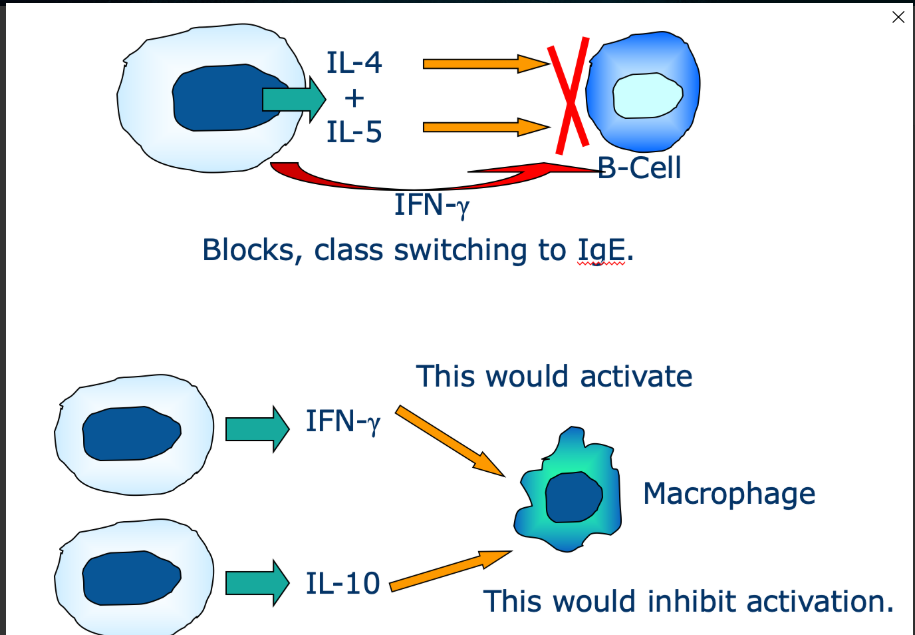
What is antagonism
One cytokine may inhibit the activity of another
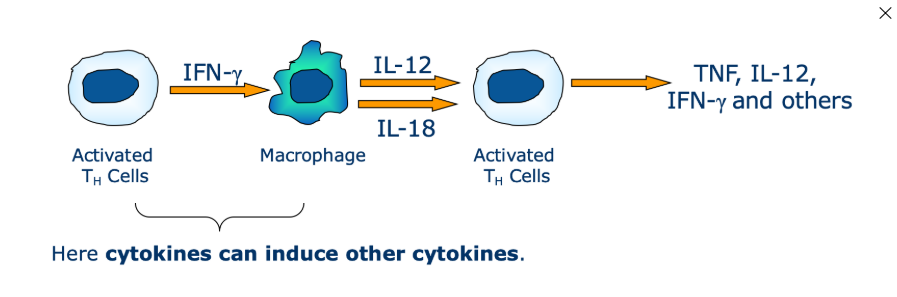
What is cascade activation
one cytokine may induce a cascade of cytokine expression involving multiple cells
How can we describe how cytokines work
a network
rarely act alone
responses often involve numerous cell types and a range of cytokines
How to cytokines regulate inflammation
They’re either pro/anti-inflammatory
Give example of both pro and anti-inflammatory cytokines
Pro = TNF(alpha symbol), IL-1, IL6, CXC-8/IL-8
Anti-inflammatory - IL-4 and IL-10
what is an inflammatory response
an innate immune reaction that ensures immune cells and other substances are brought to the affected area
why do we have inflammation responses
foreign organisms may be destroyed or inactive
injured tissue/cell remnants may be removed
tissue pair is initiated (need to consider during surgery)
what causes inflammation
causes - infection, heat, chemical substances/burns, mechanical inury, other
disorders - asthma, allergies/hypersensitivites, obesity, osteoarthritis, chronic prostitis, autoimmune diseases, diabetes, inflammatory bowel disease
what is acute inflammation
normal response, what you’d expect to deal with
repair or damage of removal of infection removes the stimulus i.e. you’ve had a thorn/break in skin, there’s an accumulation in the area of inflam, then it returns to normal state.
examples - microbial infections, hypersensitivity, physical (burn/ UV light), necrosis)
Outline chronic inflammation:
what is it
when may it occur
what does it mean
what are 3 examples
progression of acute inflammation if healing process doesn’t occur because of foreign body or continuing infection
may occur when no obvious foreign body is present
means you have weeks, months maybe years of an inflammatory process
examples - persistent infection
persistent presence of foreign bodies
immune mediated disease
what are the effects of inflammation
rubor (redness),
calor - heat
tumor - swelling
dolor - pain
lack of function
Outline how acute inflammation work on a mucosal surface
At the site of infection cytokines are released/signalling molecules that stimulate neutrophils
Neutrophils release cytokines which stimulates other resident cells
Mast cells release histamines and other vasoactive substances that increase vascular permeabitlity
acute phase reactants such as chemokines attract neutrophils and monocytes. These migrate out of the blood vessel to the site of infection
The infection is dealt with
The inflammation response is ‘turned off’.
How is inflammation initiated
At the source of infection epithelial cells and resident macrophages release chemokines and cytokines (e.g. CXCL8/IL8)
These chemicals attract other cells e.g. First migratory WBC to the site will be neutrophils, then blood derived macrophages.
This leads to a greater response
Give 7 examples of pro-inflammatory mediators, what they’re released by and what they do
released by stimulated epithelial cells
IL1(beta) - activates vascular endothelium, local tissue destruction, increases access for the effector cells and stimulates IL-6 production
TNF (alpha) - activates vascular endothelium, increases IgG entry, complement and fluid drainage to lymph
IL6 - lymphocyte activation, increased antibody production
These all lead to infiltration of leukocytes
CXCL-8 - attracts neutrophils, basophils and T-cells to the site of infection (leads to attraction of leukocytes)
Prostaglandins and Leukotrienes - stimulation of vascular smooth muscle cells and neurones (leads to vasodilation and pain)
IL12 - activates NK cells, induces differentiation of CD4 T cells into T(subscript)H 1 cells (leads to activation of adaptive immunity)
IL8 attracts phagocytic cells to the area
What often causes more damage during an inflammatory response?
The body’s own response
Some cytokines/chemokines cause damage to the body, e.g. IL1-beta so that the necessary cells (leukocytes) can infiltrate the area to stop infection.
What are some systemic effects of pro-inflammatory mediators
fever (raised temperature will help kill bacteria, as they cannot usually survive the high temperature)
Mobilisation of metabolites
Induction of acute phase proteins
What do phagocytes do in the inflammatory response?
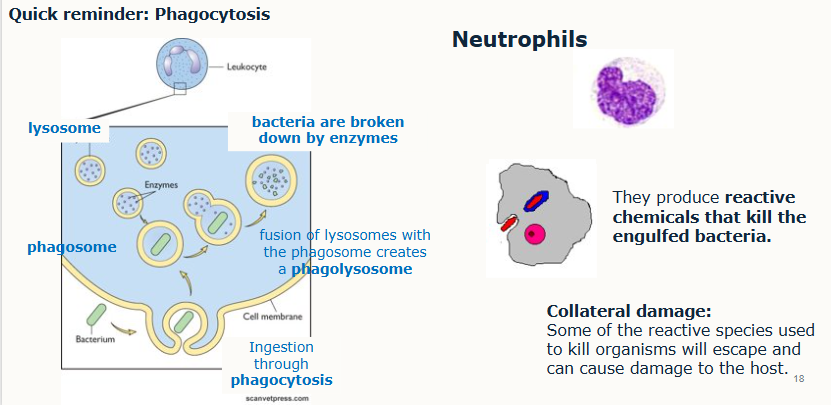
Outline the inflammatory cascade
inflammatory cell infiltrate produces more cytokines and chemokines
more resident cells become activated and more cells extravasate (including lymphocytes)
amplification of immune response leading to clearance of antigen.
Outline the role of mast cells and how we can recognise them
On activation:
rapidly release their granules and inflammatory mediators into the interstitium
preformed mediators (from granules) - histamine (2-5pg/cell), proteoglycans (heparin, anticoaglulant), serine proteases (digest specific protein bonds)
newly formed lipid mediators (eicosanoids), prostaglandin D2 (can constrict airways and dilate blood vessels), leukotriene C4
cytokine release
Recognise - characteristic secretory granules (look grainy)
present in the Interstitium (spaces b/w cells/tissues)
can be activated by the initial inflammatory response
What are the effects and what does the release of histamines lead to
effects:
dilation of blood vessels
permeability of vessels increases
activation of the endothelium (change of properties)
Leads to:
warmth
redness
local oedema
attraction of other inflammatory cells to the site of release
what are some clinical signs of histamine release
irritation of nerve endings leading to itching/pain
‘flare and wheal’ reaction - bumps and redness immediately following a mosquito bits/allergy testing (can happen after seconds)

what are the differences between mast cells and basophils
once mast cells have released histamine and other chemicals, these cause other immune system cells called basophils to prolong the reaction, damaging tissue.
Mast cells - tissue cells, release histamine, major role in asthma
Basophils - blood, release histamine, major role in anaphylactic shock
What is tethering and rolling?
blood vessel endothelium, local to the area of infection will express new receptors called SELECTINS (integrin receptors)
These cause certain leucocytes in the vessels to express INTEGRINS which can bind to the receptors
this binding slows down the leukocytes near to the area of infected tissue, this leads to their immobilisation
What happens after ‘tethering and rolling’
DIAPEDESIS
immobilised leukocytes reorganise their cytoskeletons
changes the cell shape and they ‘spread out’ over the endothelium
leukocytes pass between the gaps in the endothelial cells.
What are acute phase reactants/proteins and give 3 examples
plasma proteins
produced in the liver in response to cytokines secreted during inflammation - up to 100fold increase in their concentration
markers for systemic inflammatory response - non-specific
examples
C-reactive protein (IL6 response)
fibrinogen (IL6 response)
Serum amyloid A protein (IL-1 and/or TNF alpha response)
How do we resolve inflammation and what is it called?
Reduced presence of antigen reduces cytokine/chemokine production which leads to anti-inflammatory cytokines and inhibitors inhibiting the production and effect of pro-inflammatory cytokines, then the healing stage and return to homeostasis.
returning to a steady state
what are anti-inflammatory cytokines and give 2 examples
help control the pro-inflammatory response
act in concert with specific cytokine inhibitors and soluble cytokine receptors to regulate the immune response
physiological role in inflammation
pathologic role in systemic inflammatory states
examples are IL-4 and IL-10
What is the difference between acute and chronic inflammation?
Acute - there’s a resolution following the immune response and homeostasis is reached.
Chronic - there’s no resolution and the immune response and inflammation are excessive
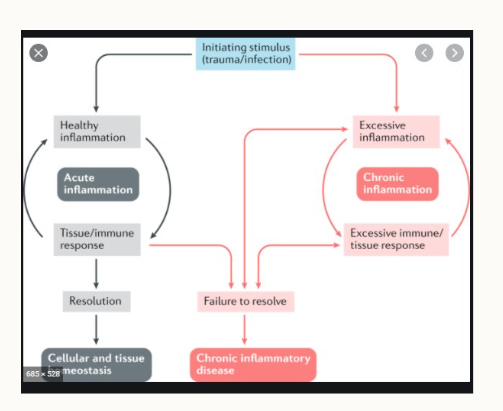
What happens if the inflammation is not ‘switched off’?
granulomas can form as a result of chronic inflammation
accumulation of immune cells
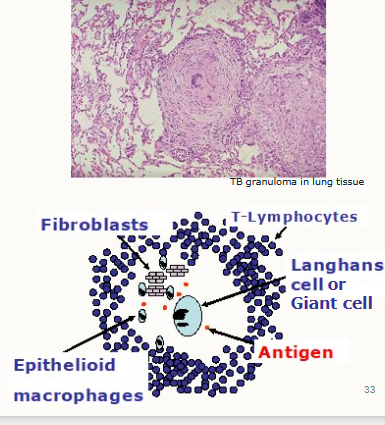
Outline granulomas:
what are they
size?
2 essential components are…
aggregates of chronically stimulated inflammatory cells
millimetre size range in tissue (can be detrimental in tissues such as lung)
confluent, forming larger areas
essential components are:
collections of modified macrophages called epithelioid cells, which when merged together (more than one epithelioid cell) forms a Giant cell/Langhans cell
a surrounding zone of lymphocytes.
Why can granulomas be dangerous in immunocompromised individuals?
For pregnant/old/very young/immunocompromised individuals, if the granuloma ruptures, this can result in an active infection if the pathogen has not been destroyed.
How to Granulomas form?
macrophages act as scavengers engulfing small particles.
The macrophages have persistent stimulation which summons more macrophages.
Prolonged stimulation results to macrohpages fusing which forms the ‘giant/langhans cells’
What main leukocyte would you expect to see in the stifle joint fluid of an animal with septic arthritis
mostly neutrophils, small amount of monocytes and if the infection is severe maybe even the pathogen itself.
Summarise the role of inflammation
necessary immune process
increases the efficiency of effector immune cell contact with foreign bodies
inflammation may be acute/chronic
cytokines place an essential role in its development as well as in dissolving inflammation to allow healing
chronic inflammation and disease may occur if the acute inflam response isn’t controlled
ganulomas form when the immune system attempts to wall off substances it perceives as foreign but is unable to eliminate e.g. infectious organisms or foreign object
What is the role of IL1- beta and what kind of cytokine is it?
IL1(beta) - activates vascular endothelium, local tissue destruction, increases access for the effector cells and stimulates IL-6 production
pro-inflammatory cytokind
what is the role of TNF alpha and what kind of cytokine is it?
TNF (alpha) - activates vascular endothelium, increases IgG entry, complement and fluid drainage to lymph
Pro-inflammatory
What is the role of IL6 and what kind of cytokine is it?
IL6 - lymphocyte activation, increased antibody production
pro-inflammatory
These all lead to infiltration of leukocytes
what is the role of CXCL-8 and what kind of cytokine is it?
CXCL-8 - leads to attraction of leukocytes - attracts neutrophils, basophils and T-cells to the site of infection
pro-inflammatory
What is the function of prostaglandins and leukotrienes and what kind of cytokines are they?
Prostaglandins and Leukotrienes - stimulation of vascular smooth muscle cells and neurones (leads to vasodilation and pain)
pro-inflammatory
What is the function of IL12 and what kind of cytokine is it?
IL12 - activates NK cells, induces differentiation of CD4 T cells into T(subscript)H 1 cells (leads to activation of adaptive immunity)
pro-inflammatory