soil fertility
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Much of the changes in grain production can be attributed to the use of fertilizers such as
N, P
the 3 strategies for managing nutrients
response:
deficiency
sufficiency
immediate profit
maintenance
removal
replenishment
long-term stability
build-up
long-term gains or improvement
perspectives of precision agriculture on why and how handle nutrients
response - profit oriented, so nutrient in sites or fields with high response
risk aversion - nutrient in the fields or areas with probable high producitvity
maintenance - replace removals by balancing inputs and outputs over time
building up - increase nutrient in areas with long-term potential
environmental - reduce environmental impacts in specific areas, fields or seasons
law of the minimum (AKA Liebig’s law)
plant growth will be constrained by the nutrient whose uptake is lowest with respect to that needed by the plant
law of diminishing returns (Mitscherlich’s law)
the two steps of cell growth
cell division (mitosis), cell expansion (turgor from water pressure)
Nitrogen
most frequently deficient
mobile
chlorosis in older leaves
phosphorus
next most frequently deficient
energy storage and transfer
potassium
cation in enzyme activation, water relationship, energy relations
resistance to disease
calcium
cell membranes, cell elongation and division
deficiency impairs emergence and unfolding of new leaves in corn
immobile
magnesium
chlorophyl, ribosomes, phosphorylation
deficiency —> interveinal chlorosis in older leaves
mobile
sulfur
immobile
typical concentrations of essential macronutrient elements in mineral soils and plants
N - 1.5%
P: 0.2%
K: 1%
Ca: 0.5%
the essential elements
C hopkins cafe managed by mine cousin Monical
the essential elements
C HOPKNS CaFe Mg B Mn CuZn MoNiCl
carbon
hydrogen
oxygen
nitrogen
phosphorus
potassium
calcium
magnesium
sulfure
cooper
zic
iron
magnanese
boron
molybdenum
nickel
chlorine
boron
cell deveopment, pollination, protein synthesis
most frequent micronutrient deficiency
immobile
copper
polymer synthesis, electron transfer
deficiency —> yellowing, stunting, curling of younger leaves
marginal necrosis
zinc
growth regulatory sythesis
deficiency: interveinal chlorosis, necrosis in older leaves, narrowing of leaves,
iron
uptake reduced by high pH
electron transfer, chlrorophyll synthesis, nitrogenase
deficiency —> interveinal chlorosis in younger leaves
toxicity due to excess accumulation is possible under low pH
manganese
generates H+, e-, O2 in Ps; e- transfer
deficiency: speckled interveinal chlorosis
toxicity due to excess accumulation possible under low pH
nickel
component of urease enzyme
lack of Ni —> urea accumulation (toxic)
molybdenum
nitrate reductase
nitrogenase
chloride
osmotic and cation neutralization
Ps
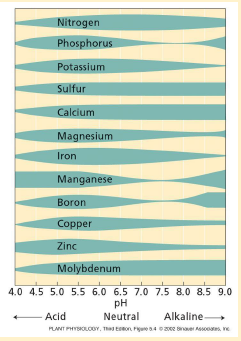
pH effects on availability of several micronutrients in soils
neutral to basic: Fe, Mg, Zn, Cu, B decrease in solubility
molybdenum increases in solubility at higher pH
beneficial/funcitonal elements
Na - partial sub for K as osmotic regulator
cobalt (co) - required for symbiotic N fixation in legumes and synthesis of B12 in ruminants
Vanadium (V) - partial sub for Mo in N2 fixation
silicon (Si) - grass tissue.
Se - not essential
toxic elements in plants
Aluminum (Al) - common toxicity
occurs below pH 5.5, where CEC can have a base saturation lower than ~50%
legumes susceptible
liming - precipitate soluble Al (reduce toxicity). increases P bioavailability
shifts exchangeable ions
Fluor
How liming (e,g for Al toxicity) works
solubilization and disociation
neutralization of acids
flocculation (soil clay in stable granule)
aggregation, soil structure
microbial activity
sources of acidity in soils in addition to AL3+
organic matter decomposiiton
root exudation
synthetic fert
acidity in rainwater
bioavailability
when a compound is accessible to an organism for uptake or absorption across its cellular membrane
nutrient bio-availability for plants depends on:
1) the release of nutrients from the solid phase in the soil to the solution phase
2) the movement of nutrients through the soil solution phase to the roots
3) the absorption of the nutrients by the plant-root mycorrhizal system
nutrient availability (bioavailability)
quantity
mobility, spatial availability
mass flow, diffusion
root growth, surface area, mycorrhizae
root-induced changes in rhizosphere
2 steps of plant nutrient acquisition
1) root-nutrient contact
root interception
mass flow
diffusion
2) nutrient uptake into the plant
passive & active
root interception
roots encounter ions as roots grow through the soil. This can be less than 1% of the soil volume
mass flow
ions transported to the root in the convective flow of water. This convective flow of water through the soil is caused by plants transpiring water though the leaves via the stomata
diffusion
ion movement along a concentration gradient from points of higher concentration to points of low concentration (and hence closer to roots)
calculating mass flow
amount of water taken up by plant x concentration of nutrient in solution
factors driving mass flow
H2O uptake rate
climate
time of day
plant vigor and health
nutrient concentration in soil solution
buffer capacity
comparing mass flow and root interception
which one can contribute more?
mass flow
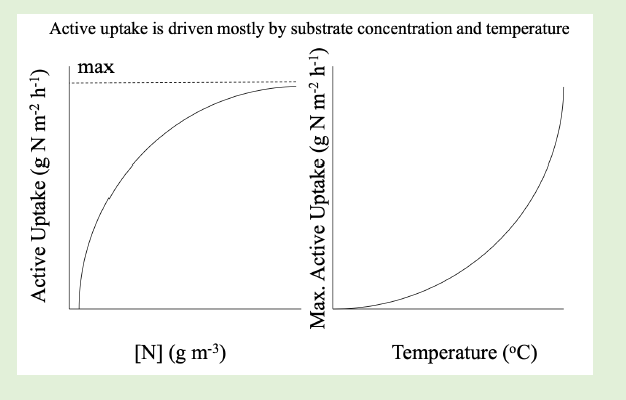
what drives active uptake?
substrate concentration and temperature
immobile plant elements
Ca
S
Fe
B
Cu
Mg
can mobile or immobile nutrietns be taken up from a larger soil volume?
mobile
passive vs active transport in cell membranes
passive: uniport channels (pores, no binding) and carriers (bind)
active ion transport: pumps that use ATP to transport ions against a concentration gradient and generate electrochemical potential
carrier proteins: 3 types of ports
uniport, antiport, symport
aquaporin
water channel in a membrane
CKC
a measure of how many cations can be retained on soil particle surfaces
buffering capacity (BC)
change in Q/change in I
ratio between changes of nutrient concentrations in soluble (I for intensity) vs solid state (Q for quantity) during nutrient additions or removals
mostly affected by solid states that exchange rapidly with soluble state (i.e adosrbed), but also by precipitated and organic states
increases with CEC, clay and SOM
the type of clay (2:1 » 1:1) is a key driver
stabilizes soluble nutrient concentrations reduces leaching without reducing availability for uptake
buffer capacity can be inferred from the slope of the adsorption curve. Steeper curves —> greater buffering
pH effects on bio-availability of nutrients
acid pH: some micronutrients (Fe, Mg, Zn, Cu, B) increase in solubility
alkaline: Mb inccreases in solubility
pH effect on macronutrient availability?
primary mechanisms of soil root nutrient contact
N: mass flow/diffusion
P: diffusion
K: diffsion
Ca: root interception
Mg: root interception/mass flow
sulfur: mass flow
micronutrients: root interception/mass flow
diffusion: Fick’s law
J = -D A dC/dx
J = diffusive flux
D = diffusion coefficent
A = area for diffusion
dC/dx = concentration gradient
soil salinity vs sodicity
soil salinity: excess salt impacting veg establishment and development
EC measured. >4 DS m-1
soil sodicity: too much Sodium (Na) relative to Ca and Mg
Ca2+, Mg2+, Na+ are measured in the solution or exchangeable phase
pH:
saline: <8.5
saline-sodic: <8.5
sodic: >8.5
all saline and sodic soils have pH >7
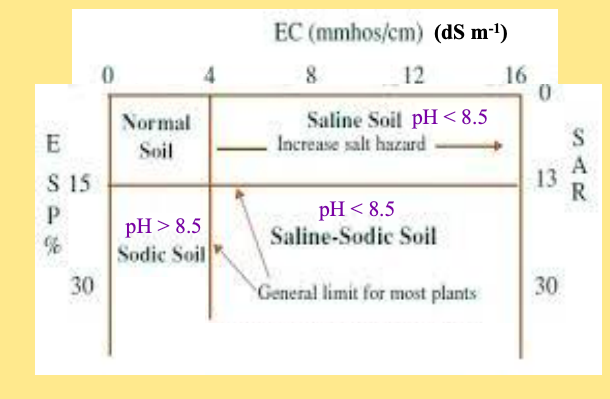
two ways to estimate sodicity
soil solution data, sodium adsorption rate (SAR(
exchangeable data, exchangeable sodium rate
where the salts come from
evaporation move and concentrate salts from deeper layers into the surface. This raise of dissolved salts in the soil profile is in part caused by upward capillary flow and the lack of deep drainage
using salty water for irrigation
recurrent fertilizer additions, incl. synthetic fert, manure
PM
location: oceans
challenges for plant growth salt-affected soils
osmosis - out of balance
water & nutrient uptake compromised
solute & water move outside of the root cells (dehydration)
root tissue collapsed (plasmolysis)
excess Cl- or Na+ is toxic to most plants
examples of plant species and their tolerance to soil salinity
very tolerant to salinity: native plant species
tolerant to salinity: barley, wheat, corn, rice
intolerant to salinity: beans, alfalfa, potato, carrot, onion
how to manage salt-affected soils
over-irrigate (can also remove nutrients)
grow native plants/salt adapted plants
inoculating plant seeds with endophytic microbes that exclude Na+
additions of gypsum (displace Na)
elemental S if there is a abundance of Ca. S —> acidity —> releases Ca