Biochem SI Session IV- Protein Structure IV
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Describe myoglobin and its function.
-Function: Oxygen storage protein in muscle
-Monomeric polypeptide of 153 amino acids
-Secondary structure consists of eight α-helices (70% helix)
-Contains a non-protein prosthetic heme group which consists of a protoporphyrin ring with a central Fe2+ atom
The iron atom in the heme group is bent out of the plane, but moves into the plane when oxygenated. Why is this?
Iron has a large electron cloud, and it moves slightly out of the plane to accommodate this. Oxygen, by binding to the iron atom, effectively shrinks its electron cloud, allowing it to move into the plane.
What amino acid’s R group provides a 5th N atom that binds to iron?
Histidine
At what coordination site does oxygen bind?
The 6th
What type of function describes myoglobin’s binding curve?
Hyperbolic
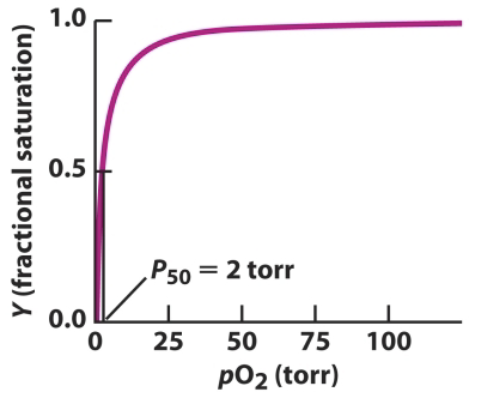
What does P50 refer to?
The dissociation constant for oxygen ligand: the concentration of oxygen when 50% is saturated (oxygenated). The smaller the value, the stronger a protein binds to the oxygen ligand.
What is the equation for determining fractional saturation?
Y=(pO2)/(pO2+P50)
pO2= O2 concentration (partial pressure)
Briefly describe hemoglobin’s structure and function.
Function: Oxygen carrying protein in blood
Structure: Tetrameric protein of four subunits: two α and two β subunits.
Can be thought of as 2 dimers with one α and one β each (α1 β1 & α2 β2)
Each subunit contains a heme prosthetic group
What are the names of the two possible conformational states hemoglobin can have in its 4º structure? How does it change?
T (tense)
R (relaxed)
α1β1 and α2β2 dimers rotate 15 degrees with respect to one another
What conformational state facilitates the binding of oxygen?
R state because the molecule is less strained, increasing the affinity of binding sites for oxygen.
Describe cooperative binding.
The binding of oxygen to one site increases the binding affinity of other sites. Unloading oxygen at one site makes unloading oxygen at other sites easier. It is more sensitive at physiological levels of oxygen.
What is the shape of hemoglobin’s oxygen binding curve?
Sigmoid
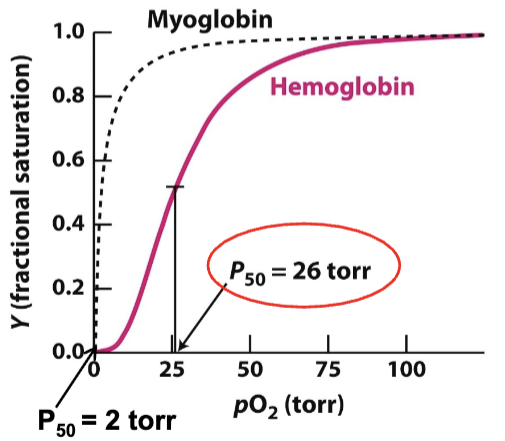
What is P50 for myoglobin?
2 torr
What is P50 for hemoglobin?
26 torr
Hemoglobin’s binding curve has a higher P50 than myoglobin’s. What does this mean?
Hemoglobin has a lower oxygen affinity than myoglobin.
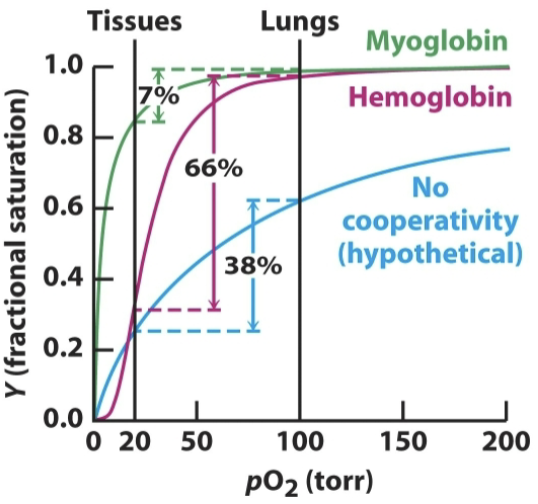
Why is oxygen affinity an important property for hemoglobin?
Hemoglobin’s function is to transport oxygen from the lungs and release it into tissues. While its lower oxygen affinity increases the concentration of oxygen needed to reach full (or nearly full) saturation, it also releases oxygen more effectively and readily when compared to myoglobin.
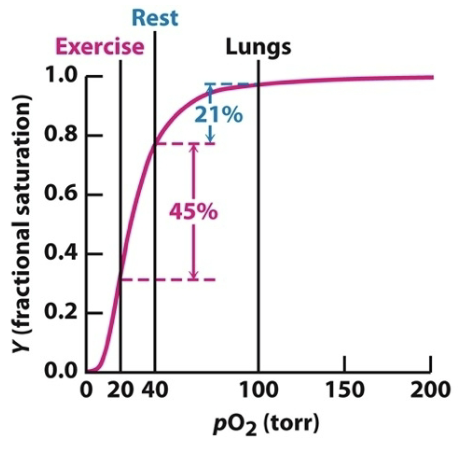
Oxygen concentrations at rest and during exercise underscore the effectiveness of hemoglobin as an O2 carrier. Explain.
The drop in oxygen concentration from 40 torr in resting tissues to 20 torr in exercising tissues corresponds to the steepest part of the observed oxygen-binding curve. This indicates that hemoglobin is fine-tuned to deliver the most oxygen when tissues are exerted.
How does oxygen binding lead to a structural transition from T to R state?
When O2 binds, iron becomes planar with the heme ring structure. This movement is transmitted to the proximal Histidine and the helix it is attached to, which is at the α1 β1 /α2 β2 interface. Structural change from O2 binding at heme is transmitted to other subunits.
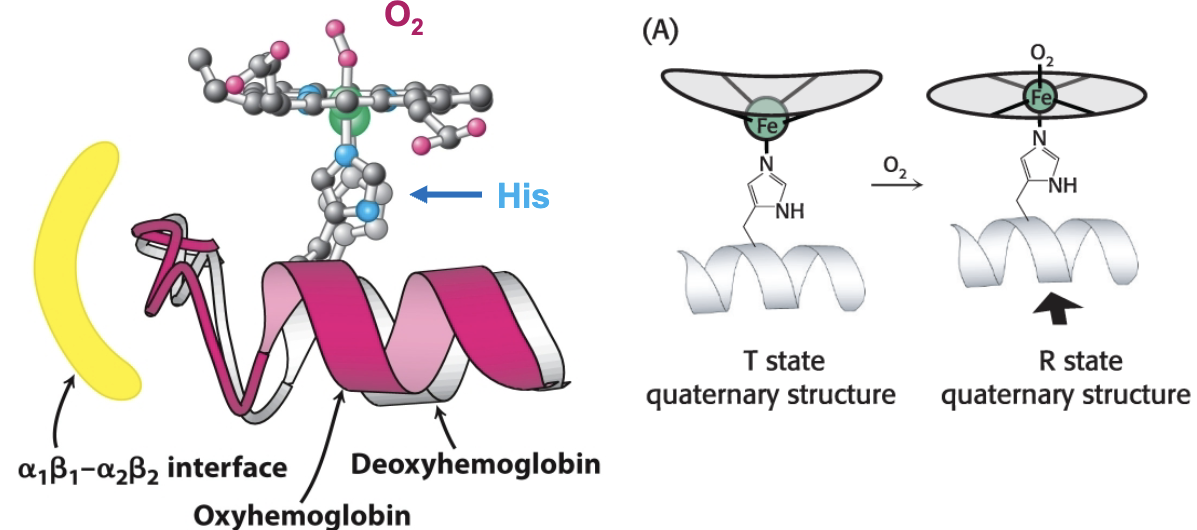
True or False: O2 can bind to both the R and T states
True- R is just more favorable when oxygen is bound/at high pO2
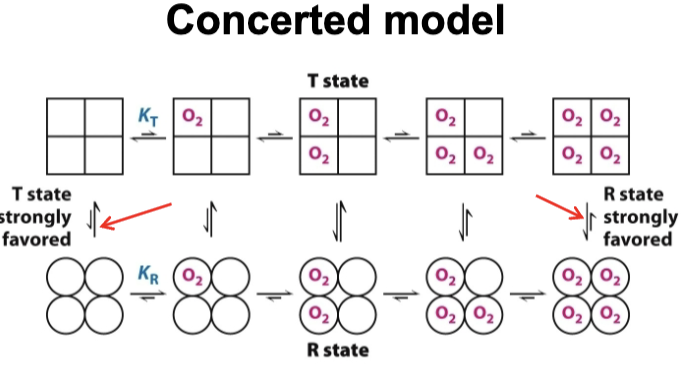
What is the concerted model of cooperativity?
It is a model that aims to describe the transition of hemoglobin from its T state to its R state. It states that all subunits are either in T or R state. At each level, there is an equilibrium between T and R states, favoring T state with no oxygen and R with oxygen binding. R has a greater affinity to O2 than T.
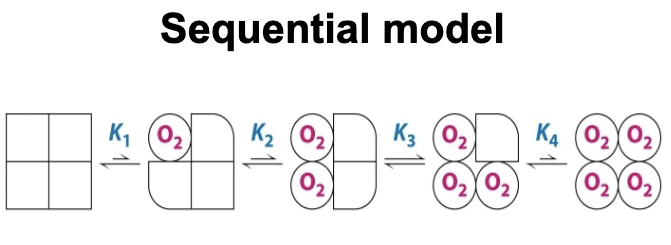
What is the sequential model of cooperativity?
It is a model that aims to describe the transition of hemoglobin from its T state to its R state. It states that the binding of O2 changes the conformation of the subunit. The conformational change induces in neighboring subunits that increase their affinity for O2.
I have an R-group that forms covalent bonds that are reduced by β-
mercaptoethanol _____
Cysteine
Why is myoglobin’s binding curve hyperbolic, while hemoglobin’s is sigmoid?
Myoglobin’s hyperbolic curve reflects independent binding at a single site, while hemoglobin’s sigmoidal curve reflects cooperative binding among subunits. This is practical, because myoglobin stores protein in muscle, so it is favorable to bind to oxygen easily and not release it easily. Hemoglobin transports oxygen to tissues, so it is favorable to be able to release oxygen more easily.
My side chain can be positively charged ______
Lysine, Histidine, Arginine
I am a hydrophobic amino acid that can have a charge in the side chain _____
Tyrosine
I am a small amino acid that is flexible and causes bends _____
Glycine
How does Levinthal’s paradox highlight the difference between theoretical folding time and real protein folding, and what does this suggest about the role of the molten globule?
The paradox explains that if protein folding were random, it would take longer to complete than the universe has existed. This demonstrates that protein folding is not random, rather it is a guided process that involves many intermediates, including the molten globule, until the protein reaches its native state. The formation of the molten globule allows for necessary non-covalent interactions to form so the protein can reach its native state
Why does the hydrophobic effect play such a critical role in protein folding, and how does it relate to the collapse into a molten globule?
The hydrophobic effect reduces the amount of entropy lost due to ordering water in a clathrate shell around individual nonpolar molecules. This proves that this is the driving force in protein folding, and it is the collapse of hydrophobic residues into the core that allows the formation of the molten globule and other stabilizing interactions.
What structural differences explain why amyloid fibrils form in Alzheimer’s disease, and how do these differ from normal folding intermediates?
Normal folding intermediates are flexible, guided, and transient steps toward the native state. Amyloid fibrils represent a misfolded β-sheet architecture that locks peptides into a highly stable and insoluble structure. This is what causes the plaques seen in the brain’s of Alzheimer’s patients.
How does the quaternary structure of hemoglobin enable cooperative binding, while the monomeric structure of myoglobin does not?
Cooperative binding refers to the phenomenon seen when a molecule binds to the heme group of a subunit in a polymer. In the case of hemoglobin, this causes the iron of the heme to move into the plane when bonded to oxygen, adjusting the attached histidine, thus pulling on the helix located at the interface. This allows for greater oxygen affinity throughout the protein. Because myoglobin is a monomer, it lacks subunits and binds independently.
Explain how the T state and R state of hemoglobin differ in oxygen affinity, and why this structural shift is advantageous in a physiological context.
The R state of hemoglobin has a greater oxygen affinity (R fav. at high pO2/T fav. at low pO2). This is advantageous in a physiological context because it allows hemoglobin to bind oxygen (R) and release it (T) to tissues very effectively.
Compare the oxygen-binding curves of myoglobin and hemoglobin. Why does hemoglobin’s sigmoid shape make it a more effective oxygen transporter?
Hemoglobin’s sigmoidal curve reflects cooperativity, allowing it to both load O₂ efficiently in the lungs and unload it in tissues. Myoglobin’s hyperbolic curve reflects tight binding, which makes it great for storage but poor for transport.
At rest and during exercise, hemoglobin’s oxygen release differs significantly. How does the steep part of the binding curve explain this adaptation?
The steepest portion of hemoglobin’s binding curve occurs between the concentration of O2 in tissues at rest (40 torr) and the concentration of O2 in tissues during exercise (20 torr). This highlights how small changes in O2 concentration can trigger large releases of O2, as hemoglobin is fine tuned to meet metabolic demands.
How do the α1β1 and α2β2 dimer rotations in hemoglobin contribute to cooperativity and allostery?
The rotation of these dimers with respect to each other allows for the T→R state transition. The R state is more favorable for O2 binding, so the rotation provides the structural basis for cooperative O2 binding and allosteric regulation.
Why would tissues with low pO₂ favor the T state of hemoglobin, and how does this ensure effective oxygen delivery?
The T state of hemoglobin would be favored in tissues with low pO2 because the T state has a much lower binding affinity for oxygen, allowing more O2 to remain released. Also, the transition from R to T state typically causes the release of oxygen, so the oxygen bound to the R state hemoglobin will be released into the tissue as it transitions to the T state.
If hemoglobin were a monomer like myoglobin, what consequences would this have for oxygen delivery in tissues, especially during exercise?
Oxygen delivery would be much less efficient, because monomers lack cooperativity. The ability of hemoglobin to transition into different states, one favoring oxygen binding and one favoring oxygen release, allows it to transport and release oxygen to tissues, with release being the most efficient during exercise. If it was a monomer, its binding curve would look closer to myoglobin’s (hyperbolic instead of sigmoidal), meaning it would not release oxygen as easily.