Chem Lab Exam 1
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
To calculate slope in excel:
= slope(y values, x values)
To calculate y intercept in excel
= intercept(y values, x values)
To calculate average in excel
= average(values)
Standard Deviation=
sqrt (value-average)²+ …/total number-1
Standard deviation function
STDEV.S
slope intercept equation
y=mx+b
Percent Error
(measured value- true value)/true value) *100
Absolute Value
actual value-measured value

100 ml beaker

50 ml graduated cylinder

10 ml volumetric flask

50 ml buret

transfer pipet

10 ml volumetric pipet

analytical balance

Pan balance

50 ml Erlenmeyer Flask

Test tube
How to measure with volumetric pipet
Attach bulb to the top
Extract liquid to meniscus
Transfer liquid
Accuracy
How close measured value is to the true or accepted value
Precision
How consistent the data is within each other
How to calculate accuracy
Absolute Error
How to calculate precision
use standard deviation, smaller value = more precise
Percent Composition in unknown mixtures
mass of component/ total mass of mixture *100
difference between gravitational filter and vacuum filter
Gravity filter uses gravity to move liquid through the filter
Vacuum filter uses vacuum to create pressure to draw the liquid through the filter
Theoretical Yield
Determine the moles of element—use molar ratio as converstion factor— Convert mols to grams
Yield Percent
Actual yield/theoretical yield *100
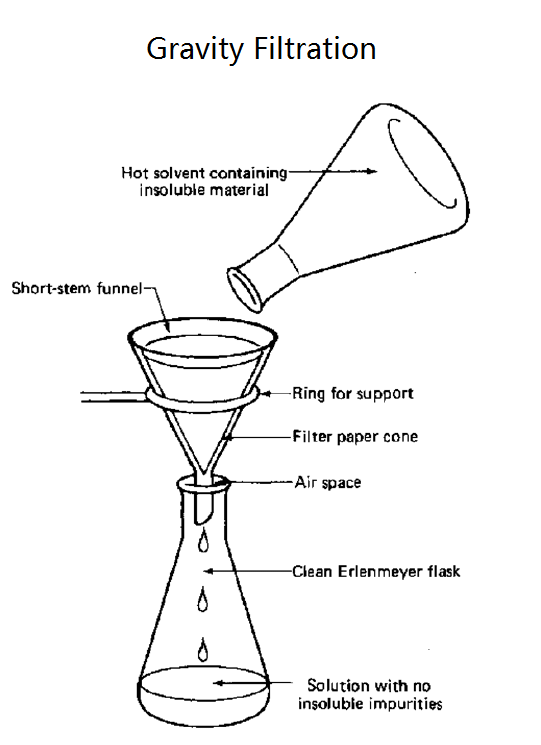
gravity filter
vacuum filter

crucible
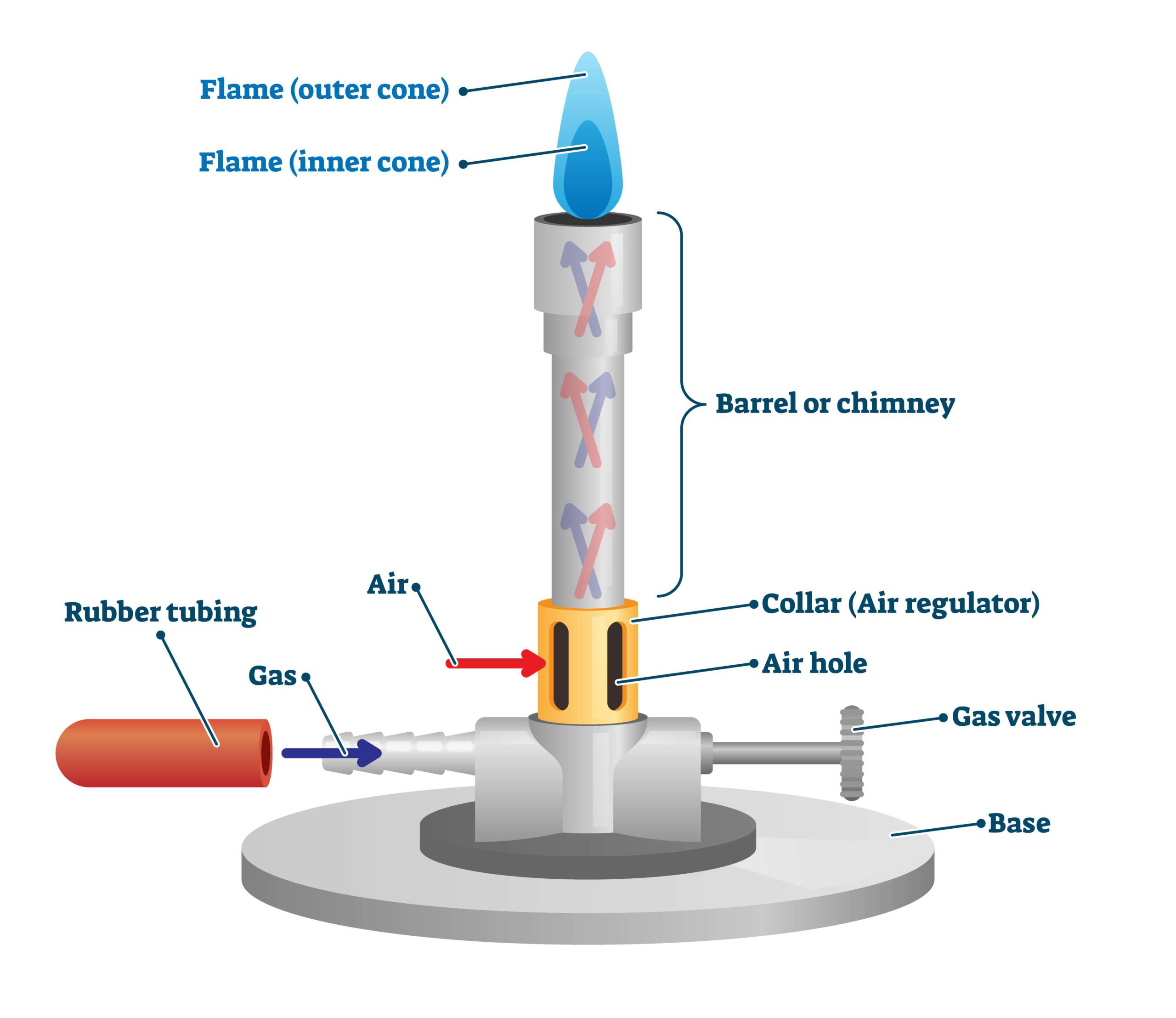
bunsen burner

watch glass
Dilution Calculation
M1V1=M2V2
Ideal Gas Law
PV=nRT
R=
0.0821
T=
Kelvin
Celcius to Kelvin: add 273.15
V=
Liters
P=
pressure, atm
n=
moles of gas
M=
mol/L
q=
(specific heat)(mass)(temp change)
Heat lost (qcal)=
qwarm water+ qcold water + qcal= 0
heat capacity=
q/change in temp (J/Celcius)