AQA A level Thermal Physics
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
What happens when a substance is heated?
The temperature of the substance rises. Causing the particles within it to gain Kinetic energy. (Therefore the internal energy of the substance increases)
What is the specific heat capacity of a substance?
The quantity of thermal energy required to raise the temperature of 1kg of a substance by 1 degree Celsius (or K) without a change of state
What is the symbol and unit of shc?
c, joules per kilogram per Celsius (J kg-1 °C-1)
Or joules per kilogram per Kelvin (J kg-1 K-1)
Is the shc the same for solids and liquids of the same substance?
No. Different substances have different specific heat capacity and when they are in different states they also have different specific capacities. For example, the specific heat capacity of ice is different to the specific heat capacity of water.
How is specific heat capacity related to how heavy something is?
The heavier the material, the more the thermal energy required to raise its temperature
How was specific heat capacity related to the change in temperature?
The larger the changing temperature the higher the thermal energy requires to achieve this change
What is the equation for specific heat capacity?

What are the changes of state?

What does it mean if a substance has a low specific heat capacity? Why?
The substance will heat up and cool down quickly as it takes much less energy to change its temperature
What does it mean if a substance has a high specific heat capacity and why?
This substance will heat up and cool down slowly as it takes much more energy to change its temperature
What does specific heat capacity help determine?
It helps determine how useful a substance would be for a specific purpose so help you choose the best material for kitchen appliances for example
Are electrical conductors good conductors of heat? Why ?give two examples?
Yes, they are good conductors of heat due to the most specific heat capacity. Eg copper and lead
What is the temperature of a substance? What is the substance has a higher temperature?
a measure of the mean kinetic energy of its particles. The higher the temperature, the more the particles move and thus the higher the average kinetic energy.
What is the lowest possible temperature on the Celsius scale?
-273 C
What happens in terms of the particles are absolute zero? Is absolute zero possible.
The particles will not move as they have no kinetic energy. Therefore, the temperature can't go any lower. Absolute zero is not possible due to the laws of thermodynamics.
What is different about the kelvin scale? (Compared to Celsius)
The kelvin scale begins absolute zero meaning there are no negative numbers in the scale. Therefore, the temperature is always directly proportional to the average kinetic enertgy over the particles
How do you convert between Celsius and kelvin?
Kelvin = Celsius + 273
A student leaves a can of lemonade (from the fridge) on the kitchen table suggest what temperature the lemonade will be after two hours
The temperature increases until it hits room temperature and it will stay at room temperature
If the kind of lemonade that was taken out of the fridge was then put in the phone container what would this mean about the temperature of the lemonade overtime?
The lemonade would stakeholder for longer as the foam container would insulate the can reducing the heat transfer to the lemonade which warms it up
Why is it better to take multiple readings instead of just one over a longer period of time waiting?
Multiple readings mean that trend lines can be drawn through many points
Explain how metals conduct heat
Particles in the metal vibrate and collide with neighbouring particles causing them to vibrate two as kinetic energy is passed on via collisions
What is the internal energy of a substance?
The sum of the randomly distributed kinetic and potential energies of the particles in a body
What is kinetic energy due to?
The speed of the molecules (energy of moving particles)
What is potential energy due to?
The separation between the molecules
What does the kinetic and potential energy of a substance depend on?
The phase of matter for example, is it a solid a liquid or a gas?
What is the symbol for internal energy and what is its unit?
U, (Joules)
What would the definition of internal energy for an ideal gas be? Why?
The sum of the randomly distributed kinetic energies only as ideal gases have no intermolecular forces and therefore no potential energy
What does it mean for particles to be randomly distributed?
The particles have different speeds and separations
What is the internal energy of the system determined by?
-Temperature (higher temperature, higher kinetic energy and vice versa E is proportional to T)
-The random motion of molecules
-The phase of matter: gases have the highest internal energy, solids have the lowest
-Intermolecular forces between the particles (greater intermolecular forces (closer), higher potential energy and vice versa) - this is linked to the phase (solid, liquid, gas) that the matter is in
How do you increase the internal energy of a system?
Doing work on it for example by increasing the pressure
Adding thermal energy by heating it
How do you decrease the internal energy of a system?
Losing thermal energy to its surroundings
Changing state from gas to a liquid or liquid to solid
Without enough insulation, the value for specific heat capacity will be incorrect. Explain whether the value calculated would be too large or too small.
Q = mcΔt
The specific heat capacity will be too large since thermal energy has lost/dissipated to the surroundings without sufficient insulation. Therefore more thermal energy is required to raise the temperature of that material by that temperature rise to compromise for the loss of thermal energy as M and Delta T stay the same. In other words, c must increase to result in a larger Q.
What is required to change the state of a substance
energy
What is the equation for specific latent heat?

What does not change when a pure substance is changing state
Temperature
What is the latent heat of a substance?
The quantity of thermal energy required to change the state of 1kg of the substance without any change of temperature.
What are the two types of latent heat?
fusion (melting) and vaporisation (boiling)
What is the relationship between mass and Latent heat?
The larger the mass the more energy will be required to change its state
What is the specific latent heat of fusion?
The thermal energy required to convert 1 kg of solid to liquid with no change in temperature
What does the latent heat of fusion apply to?
Melting a solid and freezing a liquid
What is the specific latent heat of vaporisation defined as?
The thermal energy required to convert 1 kg of liquid to gas with no change in temperature
What does the latent heat of vaporisation refer to?
Vaporising a liquid or condensing a gas
Draw and label both are cooling and heating curve for a pure substance
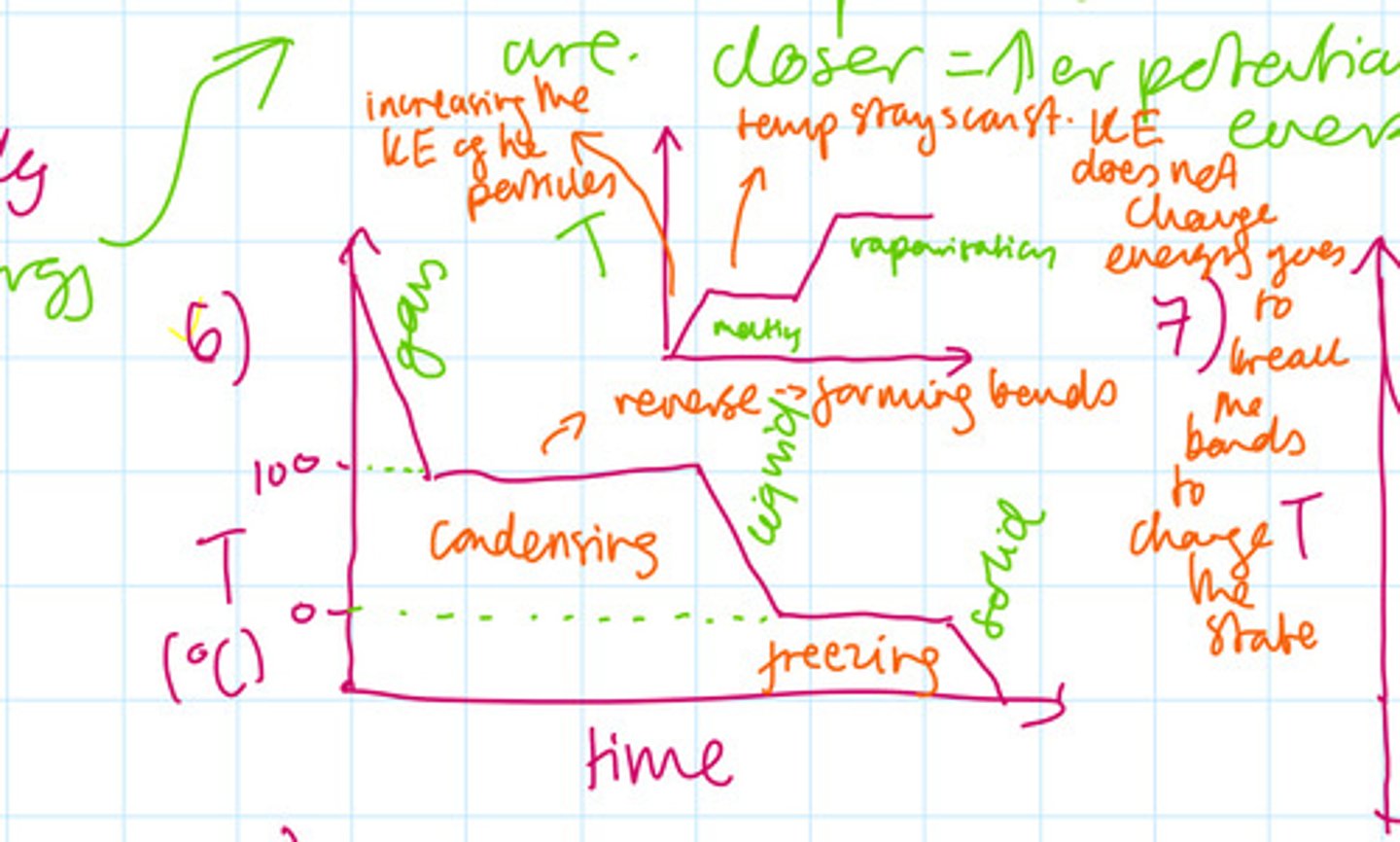
Why does evaporating 1 kg of water require roughly 7 times more energy than melting the same amount of ice to form water? In other words, why is the specific latent heat of vaporisation always much more than the specific latent heat of fusion?
When ice melts, energy is required just to increase the molecular separation until they can flow freely over each other. (Overcoming some IMFs)
However when water boils energy is required to completely separate the molecules into there are no longer forces of attraction between the molecules hence this requires much more energy . Vaporisation is also doing work against atmospheric pressure. More energy has to be supplied to separate molecules then break a solid bond.
What changes during the change of state in terms of energy?
The potential energies of the particles change, however the kinetic energy stay the same (as the temperature stays constant)
What determines the internal energy?
The temperature which determines the kinetic energy. The higher the temperature, the higher the kinetic energy.
The potential energy also determines the thermal energy and this is determined by how close the particles are. If particles are closer, then they have a higher potential energy. This is related to state (for example, gas particles are further apart than liquid. This is also related to the pressure.. higher pressure means that the particles are closer and therefore higher potential energy.
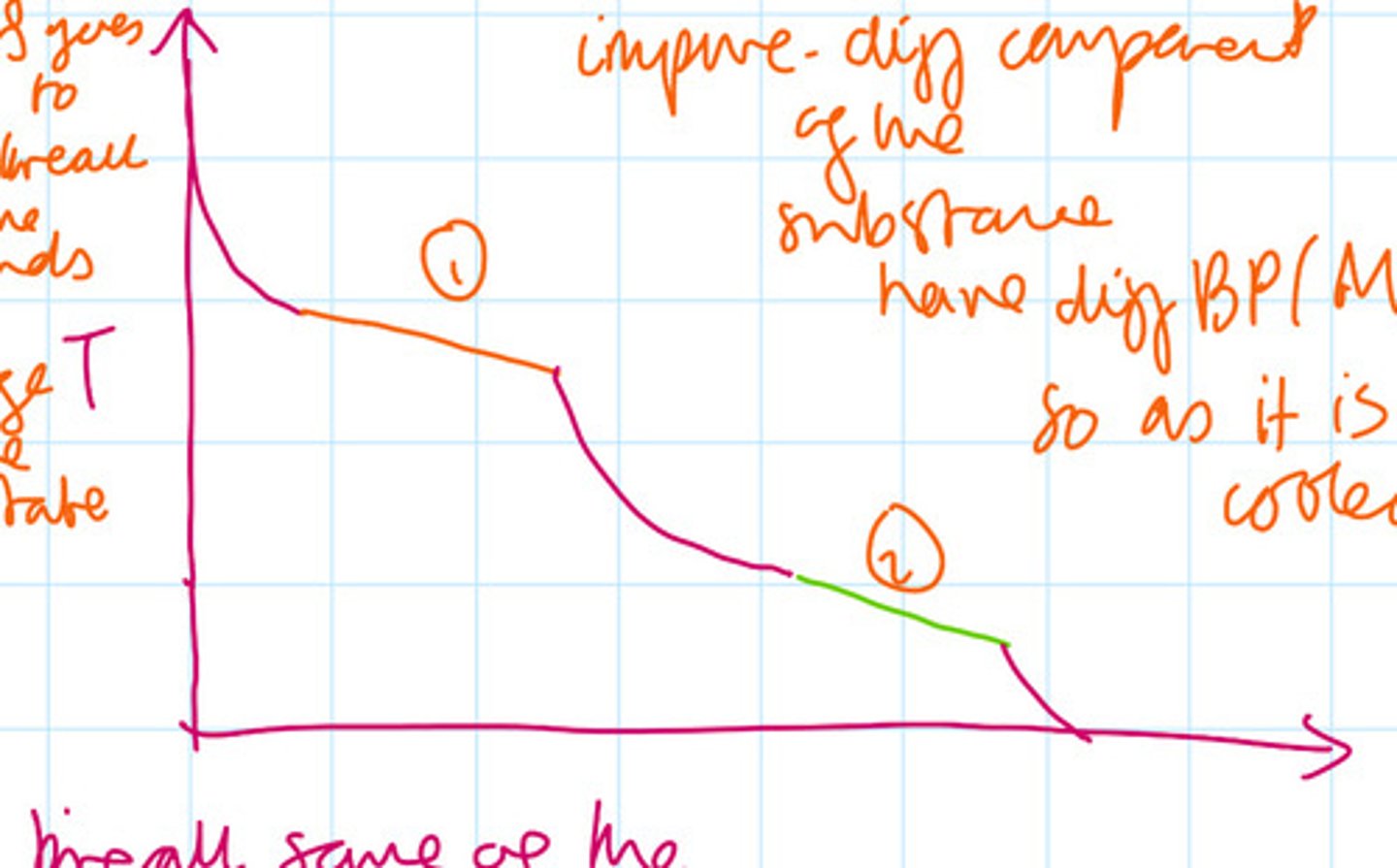
Suggest and explain a cooling curve for a non-pure substance
For impure substances different components of the substance have different boiling points/melting points so as it is called at points one and two labelled in the diagram the source of the energy is used to form the bonds while for some substances it is still cooling it
After water has boiled the temperature of the water decreases by 22°C. The mass of water in the cattle is 0.5 kg the specific heat capacity of water is 4200 J per kilogram per degrees C. Calculate the energy transferred to the surroundings from the water.
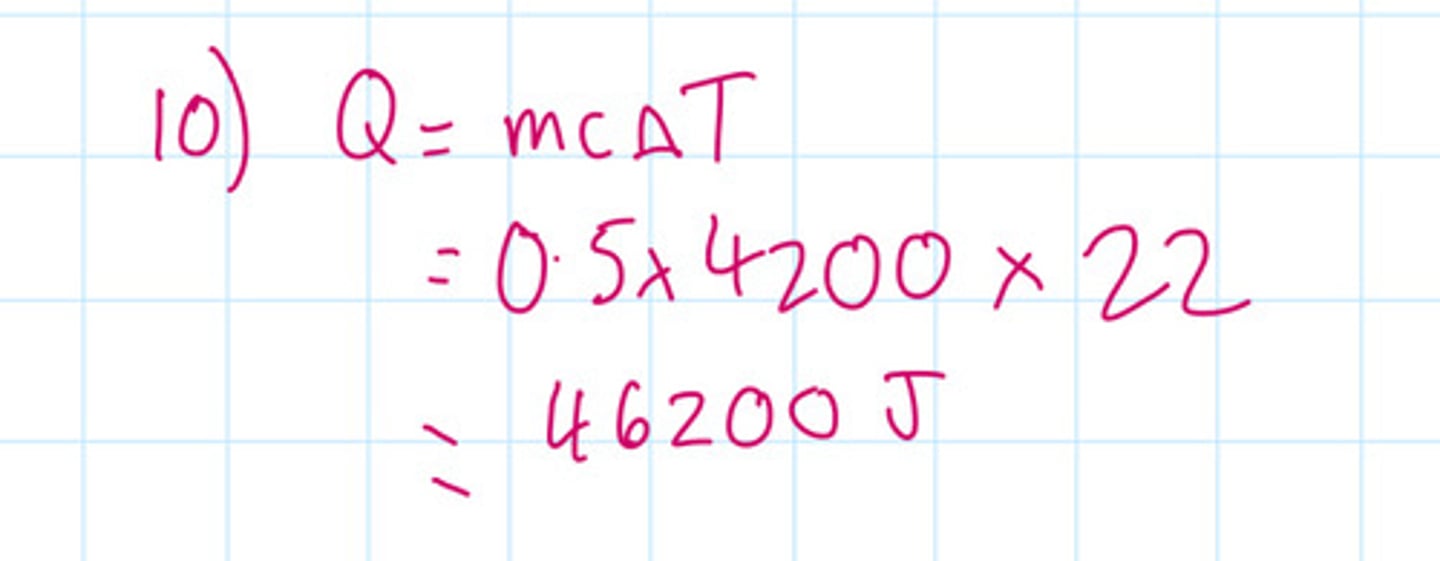
100 g of water that is already at its boiling point is boiled without any temperature change occurring if the latent heat of vaporisation of water is 2260000J/kg How much energy is needed to boil the water completely away
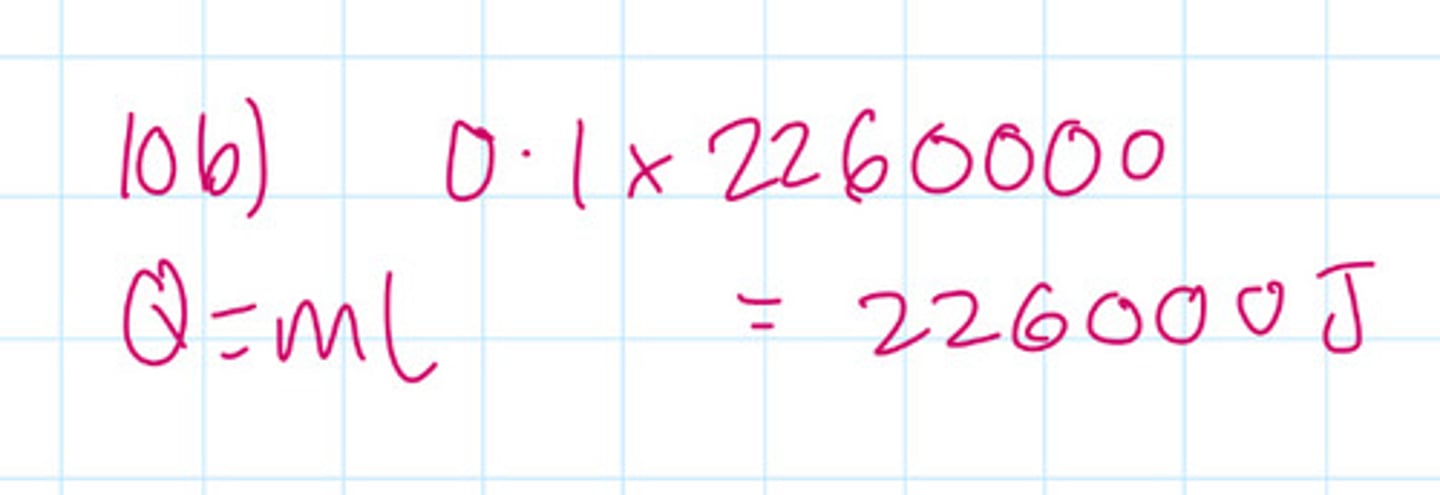
The glass of water with a mass of 0.350 kg is at 24°C. 0.033 kg ice cube is taken from a freezer set up -3°C and added to the water. What temperature will the water end up at?
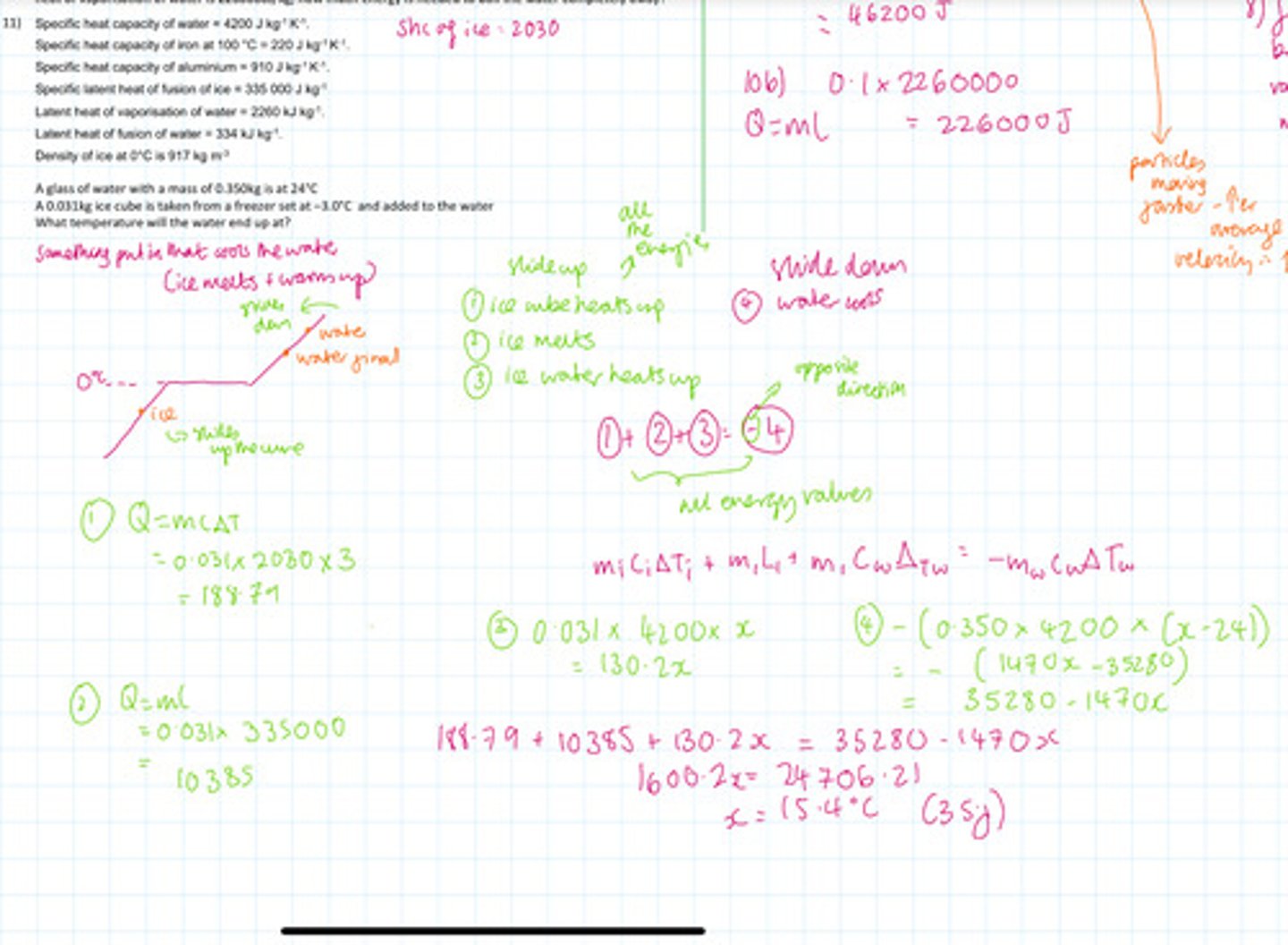
How would you calculate the energy required to boil water completely away if the water was not yet at its boiling point?
You'd have to calculate the energy required to raise the temperature to the boiling point 1st (Q = mcΔT) and then you'd have to calculate energy required to change the state of that mass of water. Then you'd add the two values together to find the total energy required.
Draw the distribution for the speed of gas particles. Label this diagram and state explain which speed value is the best to use
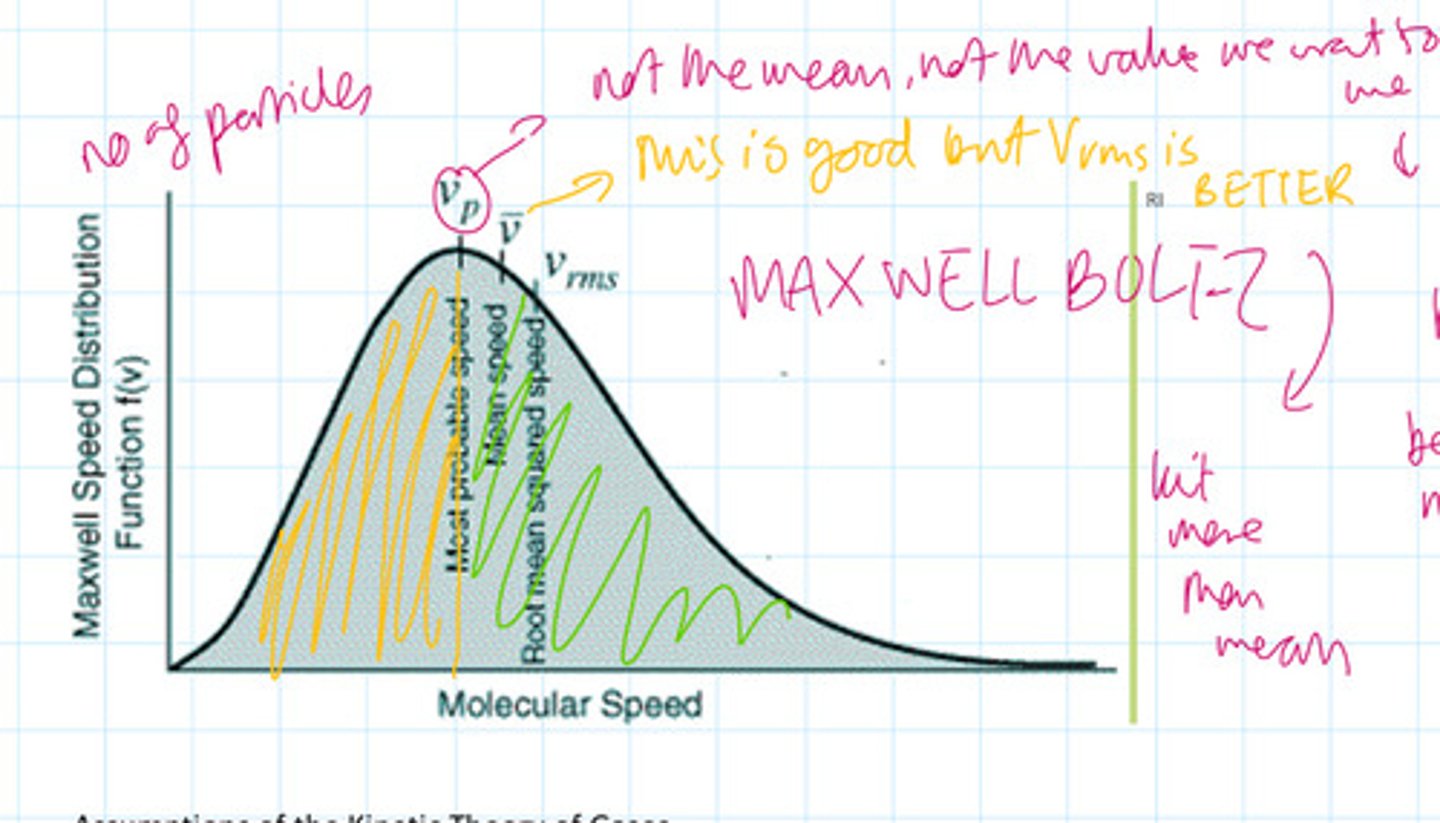
What is the RMS speed?
The square root of the mean of the squares of the speed
Why is the most probable speed not equal to the main speed?
Because the distribution does not follow a normal distribution, so the curve is skewed. See the diagram. The green and the yellow areas are not equal.
When evaluating a box of gas, why do you have to take the speed of the particles instead of the velocity of the particles?
In a box of gas, gas particles are moving in random directions a different speeds, therefore if you want to find the average velocity, this would be zero as they're moving in different directions so cancel each other out.
What does the kinetic theory of gas link?
The kinetic theory of gases models the thermodynamic behaviour of gases by linking the microscopic properties of particles (mass and speed) to the macroscopic properties of particles (pressure and volume
What are the assumptions of the kinetic theory of gases?
- The molecules of gas behaviours identical hard perfectly elastic spheres (Therefore, no kinetic energy is lost during collisions so it would not affect temperature. If all the kinetic energy was lost, the particles would not be moving and therefore would have no temperature)
- The volume of the molecules is negligible compared to the volume of the container (this is so that the particles can move around. If they were huge, there would be no space.
- The time of collision is negligible compared to the time between collisions (if the time of collision was large by F = mv-mu/t, F is very small and therefore pressure is very small.
- There are no forces of attraction or repulsion between the molecules (we do not want them interacting with each other we want them interacting with the wall)
- The molecules are in continuous random motion (to deal with equal pressure on all surfaces
- the number of molecules of gas in a container is very large, (get maxwell dist)
what is
Mean square speed (m2 s-2)
Why must all the velocities be squared in order to find the pressure of the gas?
Since particles travel in all directions in 3-D space and velocity is a vector some particles have a negative direction and others a positive direction. There are a large number of particles the total positive and negative velocity values will cancel out giving a net zero overall. In order to find the pressure of the gas the velocity must be squared this is useful as a negative or positive number squared will always be positive. To calculate the average speed of particles in a gas take the square root of the mean square speed.
What is Crms
The root means square speed (still m/s)
What does molecular kinetic theory model relate?
Temperature to kinetic energy
What are the three main steps of deriving the kinetic theory equation?
Linking pressure with volume
Adding in density
Linking pressure with kinetic energy
Step one: kinetic theory: link pressure with volume. Start with a particle in a cube length x, y, z ( are all L) ensure to only work in the x direction to start with. Derive the equation which links pressure with volume for only one particle.

With the equation you have in step two convert this so that it applies for many particles. Then add in density to the equation.
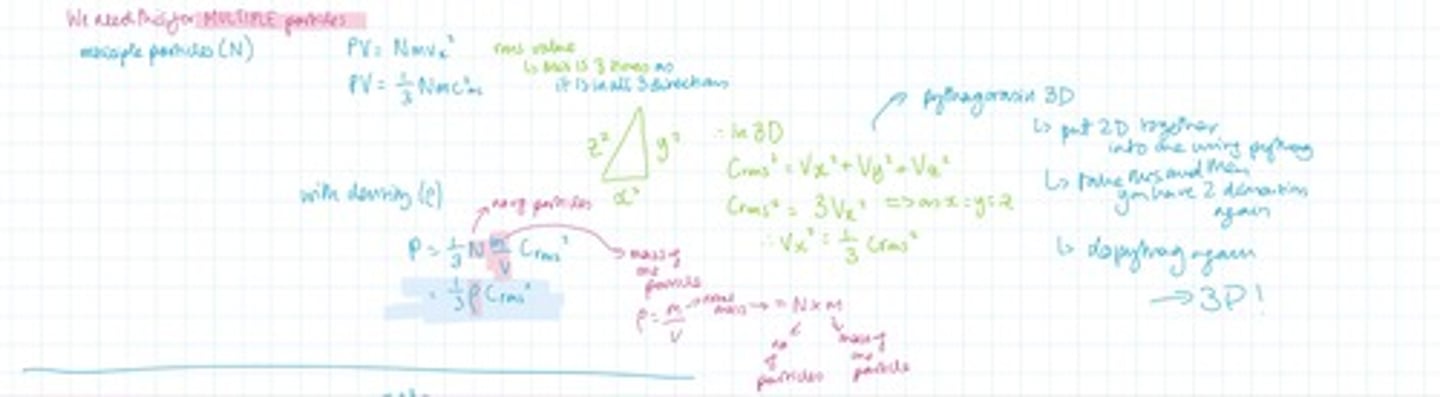
With the equation for multiple particles (not including density) can you factor in kinetic energy? What about temperature? What equation do you have to use to be able to factor in temperature?
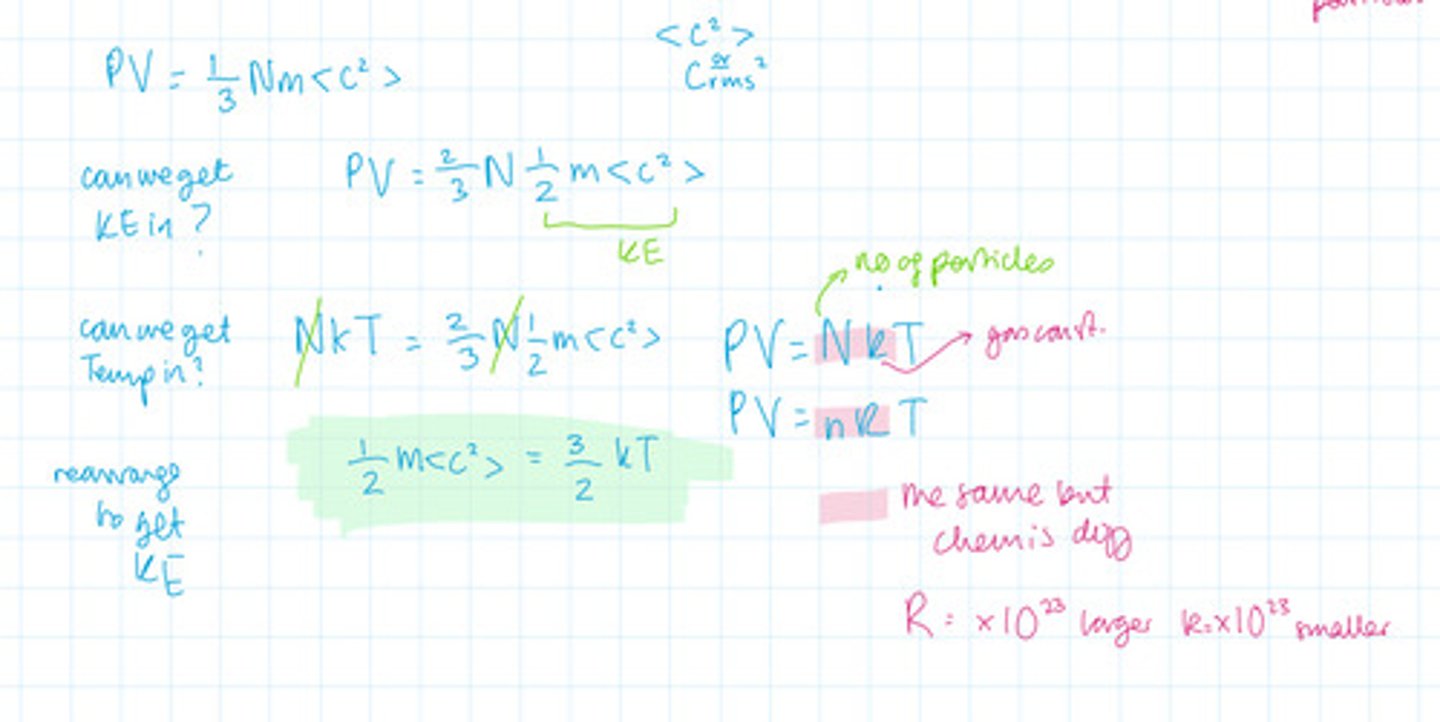
What is the relationship between number of particles and pressure?
directly proportional: the higher the number of particles the higher pressure
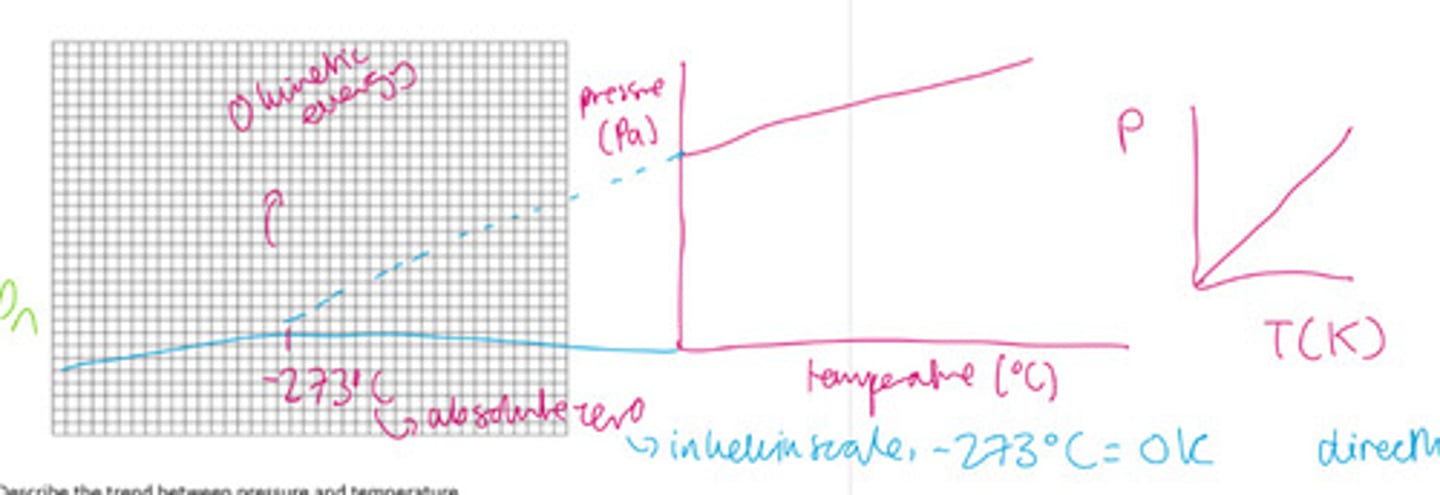
What does pressure law state?
When a gas has a fixed volume, pressure is directly proportional to the absolute temperature (kelvin).
If the temperature was in Celsius and not kelvin, the relationship would be linear, however the line would not pass through the origin. You have to extrapolate the line to the negative X axis until it crosses the X axis. This should cross the negative axis at -273°C. This is absolute zero.
Draw a graph of the relationship between pressure and temperature for both temperature in Kelvin and temperature in Celsius
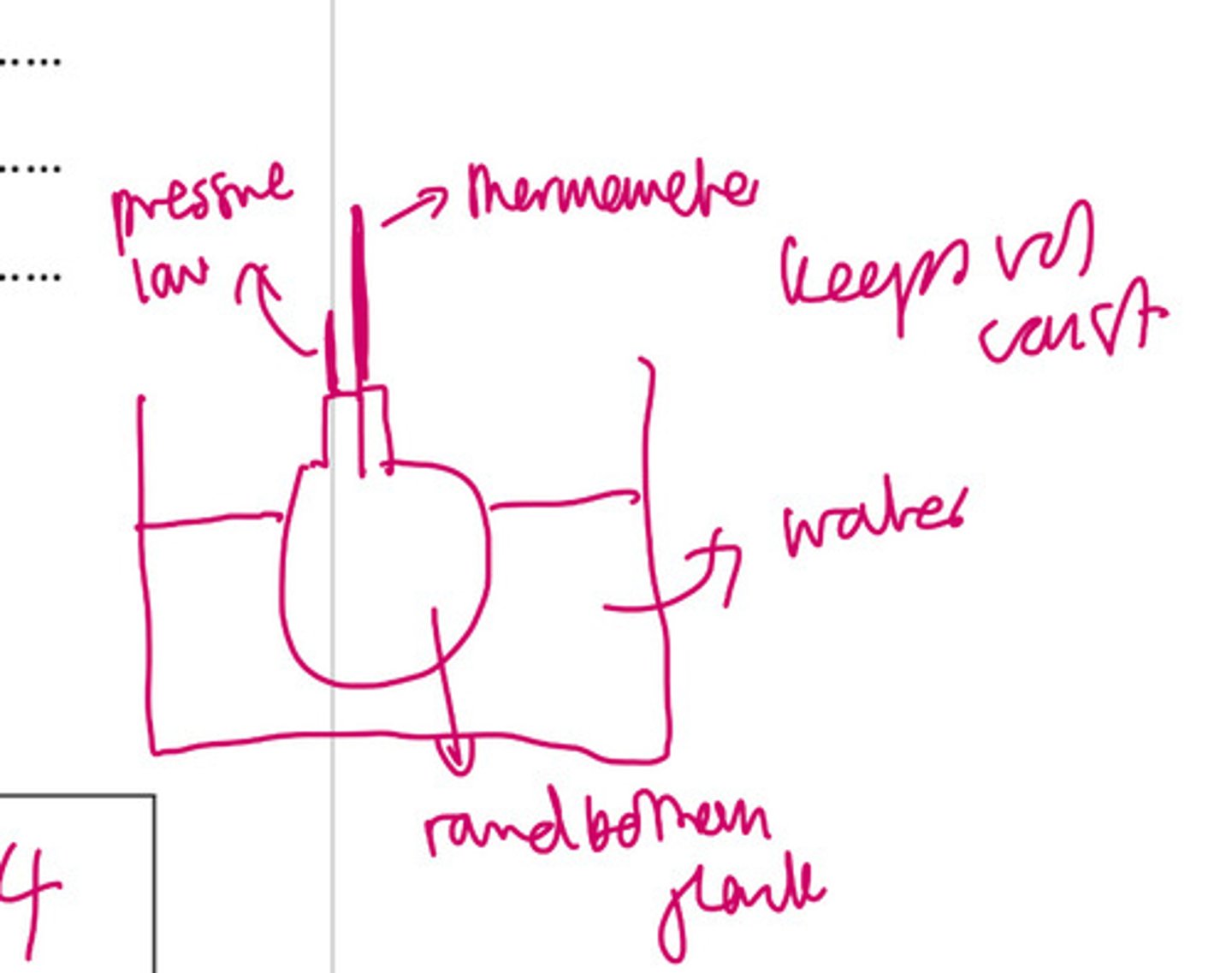
Draw the apparatus to investigate pressure law
What is the equation for pressure law?
P1/T1 = P2/T2

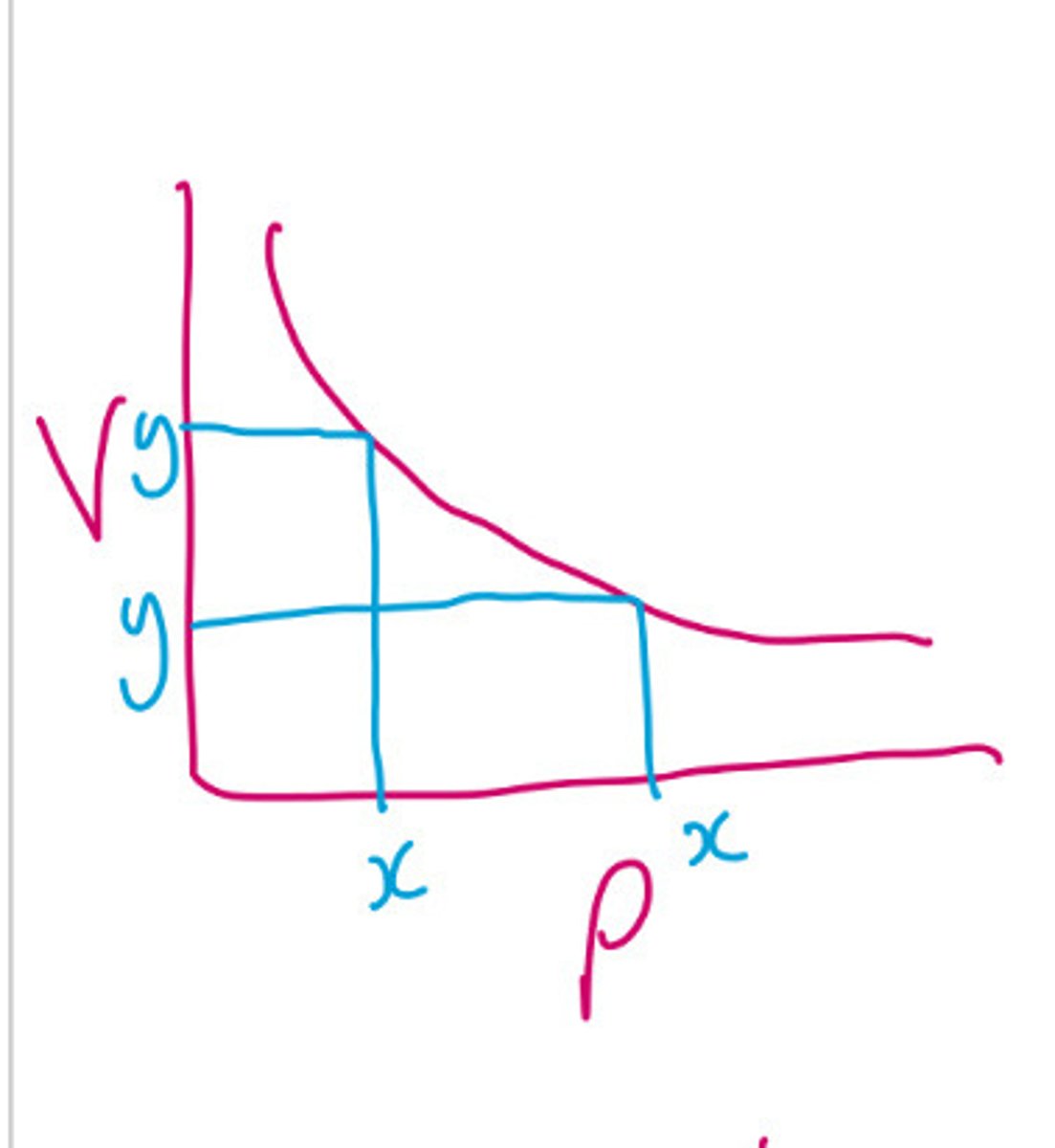
What does Boyle's law state?
At a constant temperature, as volume increases the pressure decreases they have an inversely proportional relationship
Draw the graph relationship between pressure and volume at a constant temperature
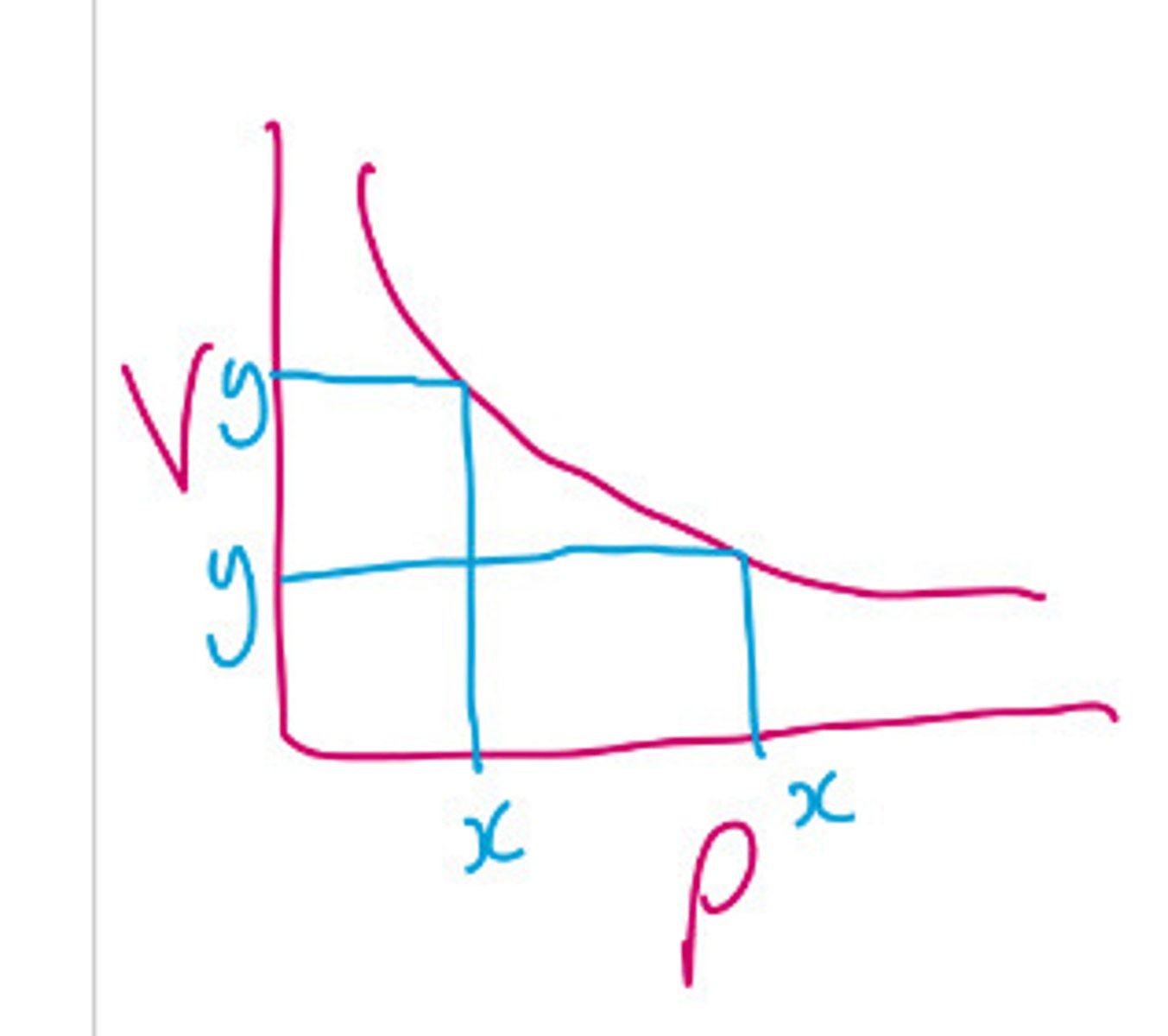
How would a boyle's law graph of a higher temperature gas compare with one of a lower temperature gas?
What is the equation from Boyles law?
PV = k
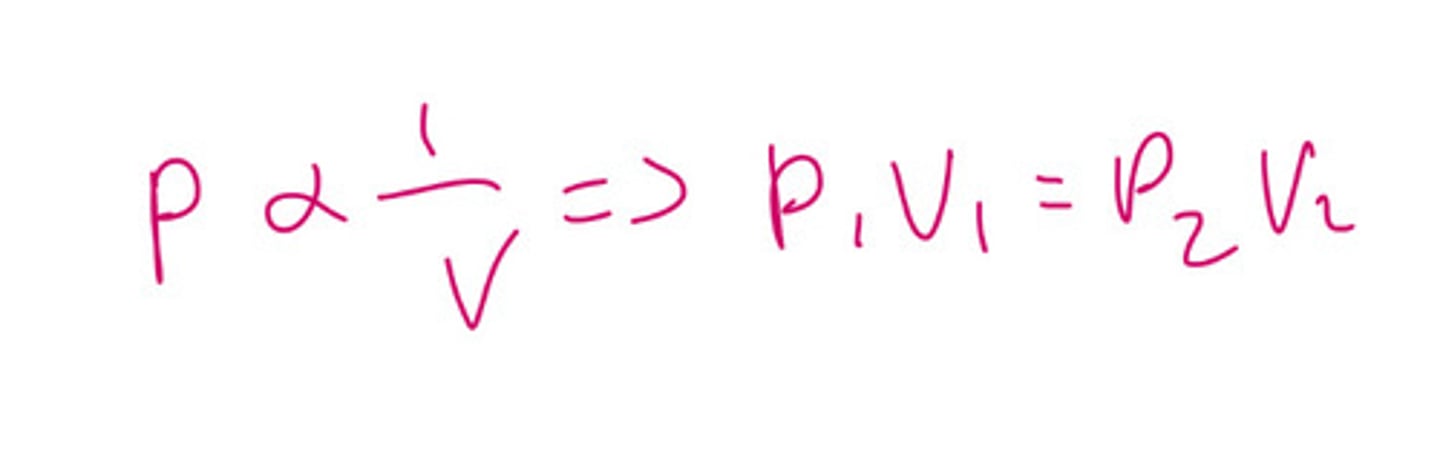
How can you tell if there's an inverse proportional relationship?
When one Variable doubles the other halves. From a graph: multiply Y by X at three points, if you get a constant it is inversely proportional
What is one atmosphere equivalent to?
101325Pa
What is one bar equivalent to?
100kPa
What does Charles's law say?
That at a constant pressure, temperature (K) is directly proportional to volume. (as temperature increases the volume increases they have a directly proportional relationship.)
Draw a graph for Charles' law
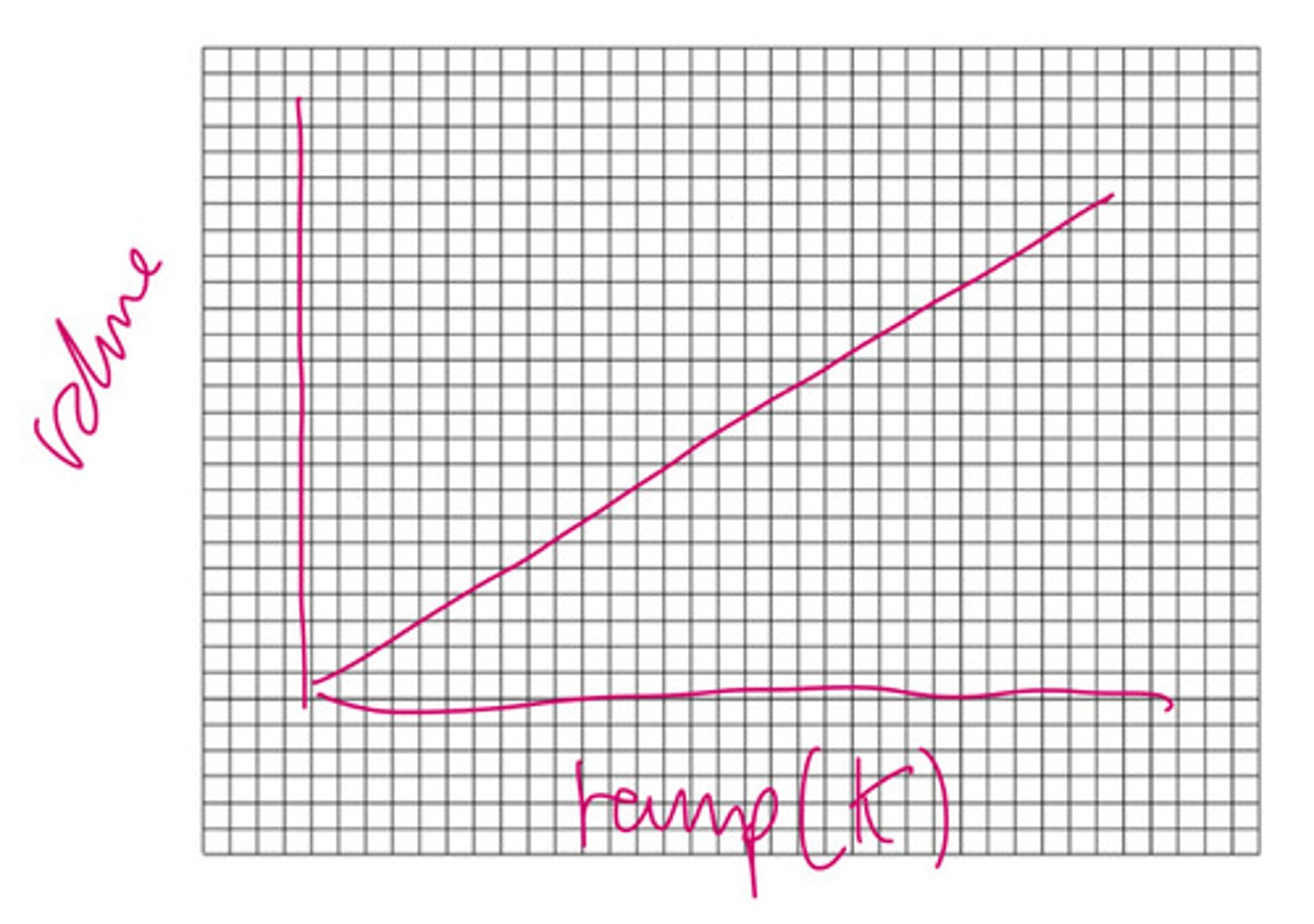
What unit is volume measured in?
m3
What is the equation from Charles law?

What is the problem with Charles's law? And what does this mean? It only applies to?
Using Charles' law, zero Kelvin the gas should have zero volume. However, this doesn't work as particles cannot have zero volume. (Therefore we must assume that the particles have zero volume and so only works for an ideal gas which uses all of these unrealistic assumptions)
Also in reality, the gas would cool and convert to a liquid at a certain point
What does Avogadro's law state?
The number of particles is proportional to the pressure
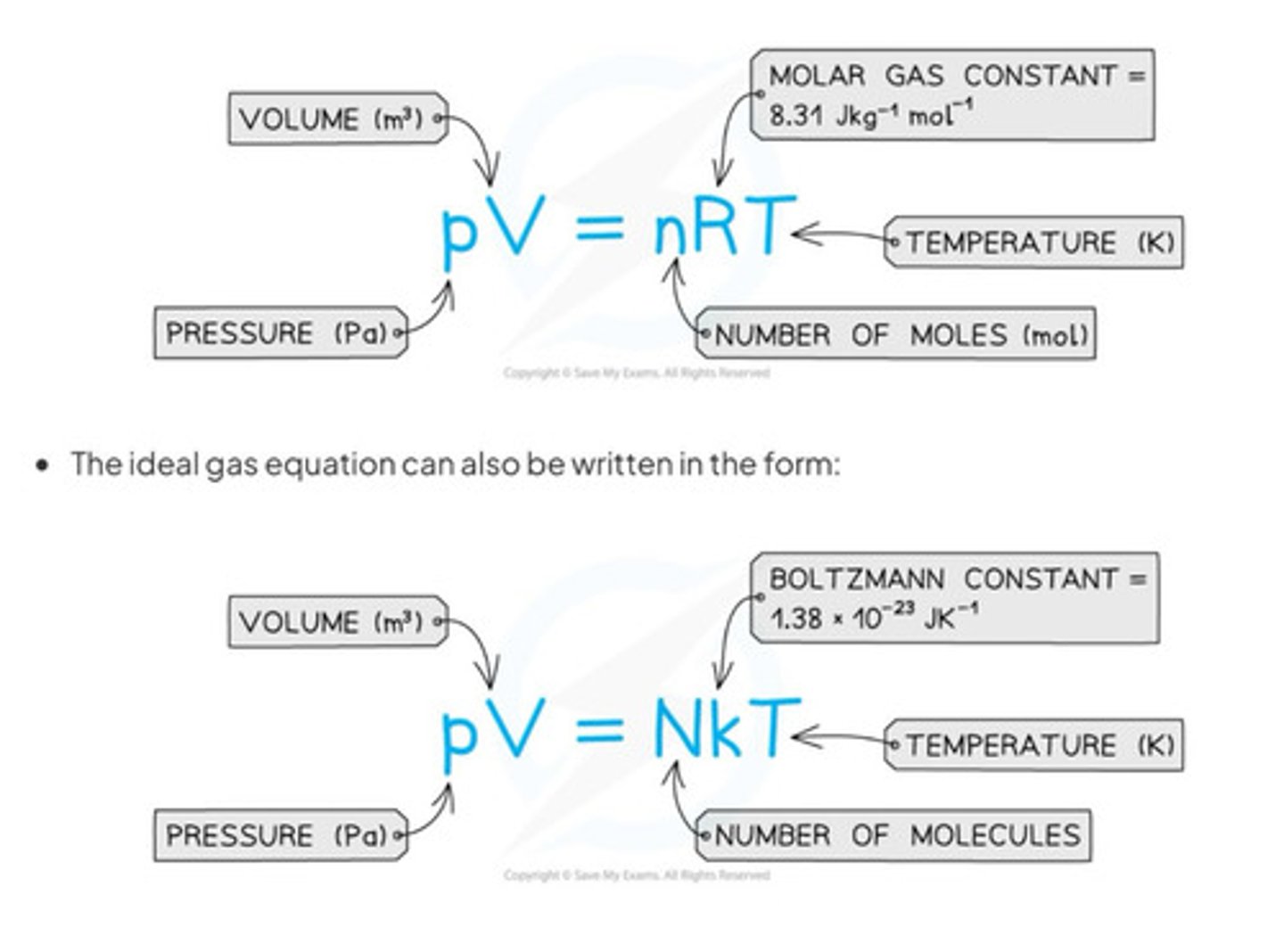
What happens when you combine all of the gas law formulae
The ideal gas equation
What is another assumption for an ideal gas?
The gas cannot be liquefied
What is an ideal gas defined as?
A gas which obeys the equation PV = nRT AT ALL PRESSURES, VOLUMES AND TEMPERATURE
Explain how gas exerts a pressure on a balloon
The particles move randomly at high speeds in all direction. These particles collide with the walls, which applies a force (change in momentum) over an area which is pressure.
Explain how gas pressure changes with temperature
The gas is heated, so the particles gain more kinetic energy. Therefore, the particles collide with the walls more frequently, With a greater force (greater change in momentum) over the same area, which is an increased pressure.
Explain how gas pressure changes with volume
Smaller volume the same number of particles collide with the walls meaning that there's the same force over a smaller area, which is increased pressure (P= F/A)
Explain why gas volume changes with temperature
When a gas is heated, the particles gain kinetic energy and move faster. Therefore, they collide more often with greater force on the container walls. Since the container can expand the gas volume increases until the pressure of the gas is back to the constant atmospheric pressure. (Also the intermolecular forces between the molecules we resulting in the expansion of spaces between the molecules)
What is a synonym for brownian motion?
Random thermal motion
What is brownian motion?
Brownie motion is when small particles such as pollen or smoke particles that are suspended in a liquid or gas or observed to move around in a random erratic fashion.
When does Brownian motion occur and why?
Brownian motion occurs because fluid molecules collide into the suspended particles. We can therefore prove the size and constant random motion of the suspended particles. (Eg smoke) think about it this way: the smoke particles wouldn't be moving on their own on a surface, therefore if they are moving when suspended in a liquid, by Newton second law, these particles must have a force acting on them to be accelerating. Therefore, the fluid molecules collide into the suspended particles so that they can accelerate.
Do the fluid particles and the smoke particles move in the same kind of motion?
No, the smoke particles move via Brownian motion . The particles collide with the smoke and therefore there is a change in momentum and therefore force acting on the smoke, causing the smoke particles to accelerate. this is the random thermal motion that we observe.
However, the air particles move randomly due to their kinetic energy they have due to their temperature. (Not brownian)
What does it mean when particles are in random motion?
The particles are travelling at a range of speed with no preferred direction of movement
Why are the collisions of the smaller particles into the larger particles successful? In other words, why do they cause the larger particles to change their speed and directions randomly?
The atoms are able to affect the larger particles in this way because they are travelling at a speed much higher than the larger particles and so they have a lot of momentum which they transfer to the larger particles when they collide. (Newtons second law equation: (F = mv-mu /T)
Derive the formula of work done on a gas

What is the definition of work done?
Transferring energy
When work is done on a gas, where does this energy go? And what is the effect?
Energy is transferred to the gas particles meeting they have more kinetic energy so they move faster and the root main square of their speed increases (internal energy increases)
What happens when a gas is compressed?
Work is done on the gas and energy is transferred to the gas particles. Volume decreases