Chapter 3 Arrangement of Electrons in an Atom
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
Energy level
An energy level is defined as the fixed energy value that an electron in an atom may have.
What is meant by the ground state of an atom?
The ground state of an atom is the one in which the electrons occupy the lowest available energy levels.
What is meant by an excited state of an atom?
The excited state of an atom is one in which the electrons occupy higher energy levels than those available in the ground state.
Notes:
- An electron cannot occupy spaces between energy levels – it is unstable and will fall to a lower energy level
- The letter n is used to represent energy level 1
Evidence for existence of energy levels in atoms
Bohr investigated the line emission spectrum for hydrogen by exciting electrons in hydrogen atoms
Explain how the visible line emission spectrum of hydrogen arises and provides evidence for the
existence of energy levels (The Bohr Theory)
(Bohr proposed that electrons have fixed (quantum or discrete) amount of energy.
Meaning an electron in an atom is restricted to having certain levels of energy.
Bohr concluded that:)
Electrons revolve around the nucleus in fixed paths called orbits.
It neither gains or loses energy when on these orbits.
They are said to be in their ground state.
If energy is provided (heat or electricity), the electron absorbs this energy and jumps to a higher energy level (orbit).
This is called the excited state.
The energy absorbed is the difference between the ground state and the excited state. E2 – E1
In the excited state, the electrons are unstable and only remain for a short period of time.
As they fall back to a lower orbit or energy level, the excess energy is released in the form of light.
As only definite amounts of energy are emitted, this implies that the electrons can occupy only definite energy levels.
The frequency of the light is equal to the formula E = hf
E2 - E1 = hf E = energy level, h = Planck's constant, f = light frequency
Give two ways electrons in an atom can be excited
1) Heating the element
2) Passing an electric current through the element
What is a line emission spectrum?
A line emission spectrum is a series of coloured lines that correspond to specific frequencies of light emitted when electrons in an element are excited
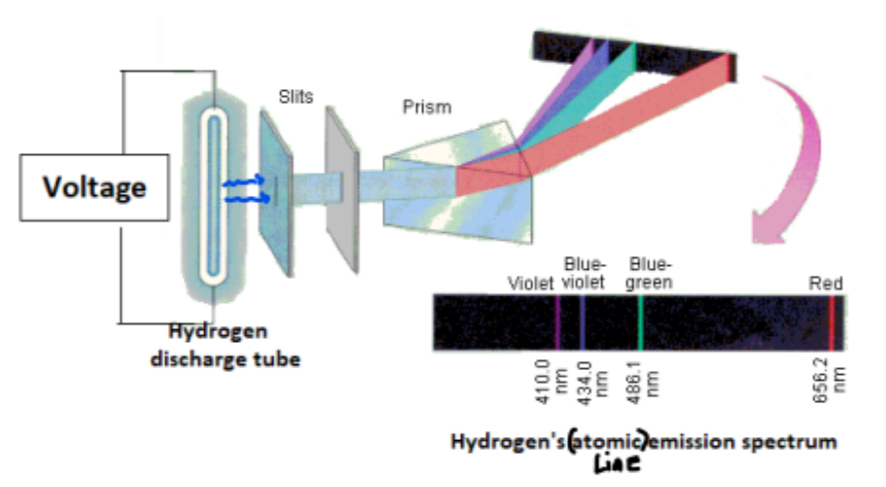
What name is given to the series of visible coloured lines produced in a hydrogen emission spectrum, caused by electrons falling from higher energy levels to n =2?
• The Balmer series
Electrons falling from n = 3 to n= 2: Red colour produced
Electrons falling from n= 4 to n= 2: Blue-green colour produced
Electrons falling from n = 5 to n = 2: Blue-violet colour produced
Electrons falling from n = 6 to n= 2: Violet colour produced
Notice:
- Electrons that fall from higher energy levels to n = 3 produce lines that are in the invisible infra-red region of the spectrum - The Paschen series
- Electrons that fall from higher energy levels to n = 1 produce lines that are in the invisible ultra-violet region of the spectrum – The Lyman series
Explain why there is no yellow line in the hydrogen emission spectrum
• There is no corresponding electron transition in hydrogen that would produce light of frequency that gives a yellow colour
Note: Each element has its own unique atomic emission spectrum
Note: Flame tests also provide evidence for the existence of energy levels in atoms
What are flame tests?
• Heating an element in a bunsen burner will cause that element to produce a characteristic colour i.e. give out light of a certain frequency
• The element can therefore be identified
Give two reasons why different elements have different atomic emission spectra/impart different colours to a bunsen flame
1) Different metals have different transitions between their energy levels
2) Different metals have different numbers of electrons - meaning specific frequencies of light are emitted for each specific element
Give three applications of the use of knowledge of energy levels in everyday life: 1) Atomic Absorption spectrometry (AAS)
• As well as atoms emitting light of specific frequency, atoms will also absorb light of that same specific frequency
• An atomic absorption spectrum is produced.
Principle:
- White light is passed through a gaseous sample of the element being analysed
- Electrons in the sample absorb certain frequencies of the white light
- These frequencies are then “missing” from the spectrum produced
Note: Because particular elements have specific energy levels, the element can be identified from the wavelengths absorbed
Uses of atomic absorption spectrometry:
a) Identifying elements present in stars
b) Detecting the presence and concentration of “heavy metals” in water
Examples: Lead, Mercury
Give three applications of the use of knowledge of energy levels in everyday life: 2) Sodium streetlights
• Streetlights use sodium vapour lamps in an excited state
• A characteristic yellow colour is produced
Give three applications of the use of knowledge of energy levels in everyday life: 3) Fireworks
Particular elements are used to give off characteristic attractive colours
Bohr's model
Bohr’s work on energy levels led him to coming up with his model of the atom as having electrons as tiny negative particles that orbit the nucleus in fixed paths or orbits/shells. Electrons in a particular orbit have a fixed amount of energy (energy levels)
Describe four limitations of Bohr’s theory that led to its modification
1. Bohr’s explanation only worked for the hydrogen emission spectrum - it failed to explain many of the lines in the emission spectra of the other elements
2. Energy levels were found to consist of further sublevels
3. Electrons were found to have properties of particles and properties of waves and could not be described as tiny particles only (particle – wave duality)
4. The exact position and velocity of an electron could not be measured at the same time so they cannot be described as travelling in fixed paths – led to Heisenberg’s uncertainty principle
What is a sublevel (energy sublevel)?
• A sublevel is a subdivision of a main energy level and consists of one or more atomic orbitals of equal energy
Note: sublevels are labelled s, p, d and f
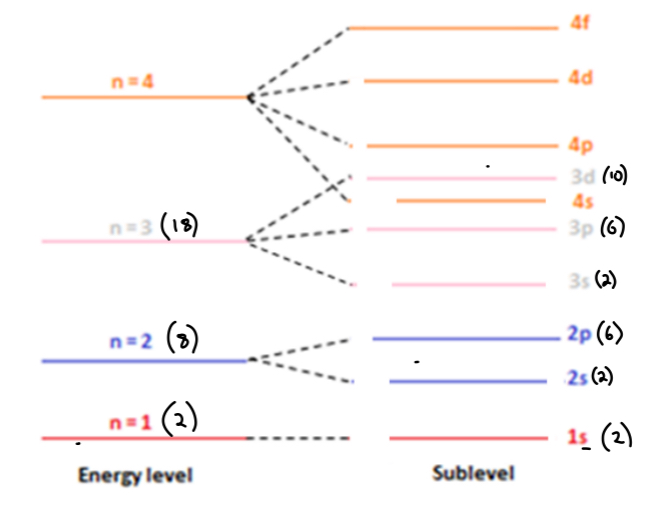
Define a sublevel
A sublevel is a subdivision of a main energy level and consists of one or more orbitals of the same energy.
Wave nature of an electron
De Broglie found that all moving electrons actually move in a wave motion. He called this wave particle duality.
Heisenberg's Uncertainty Principle
states that it is impossible to measure at the same time both the velocity and position of the atom
Define an atomic orbital
An orbital is a region in space where there is a high probability of finding an electron.
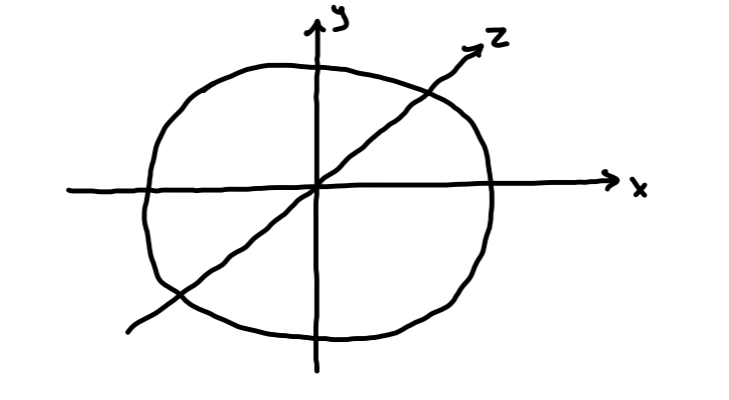
s-orbital
Spherical shape
• There is ONE s-orbital in an s-sublevel
• Each s-orbital can hold two electrons
• Therefore an s-sublevel can hold two electrons
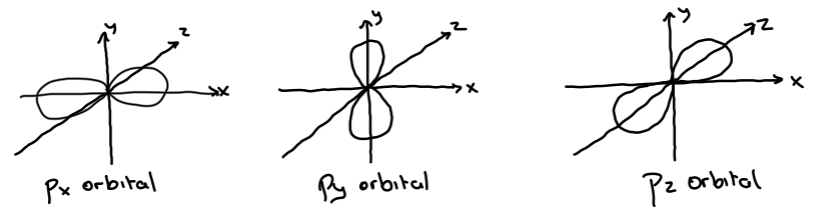
p-orbital
Dumbbell shape
• There are THREE p-orbitals in an p-sublevel
• Each p-orbital can hold two electrons
• Therefore a p-sublevel can hold six electrons (px = 2, py = 2, pz = 2)
Note: The p sublevel consists of the three p-orbitals together
D orbital
Complex shape
• There are FIVE d-orbitals in a d-sublevel
• Each d-orbital can hold two electrons
• Therefore, a d-sublevel can hold ten electrons
Give two differences between an atomic orbit, as described by Bohr, and an atomic orbital
1) Orbit - electrons travel in fixed paths, in a definite location
Orbital - electrons have wave properties, have a high probability of being found there
2) Orbit - has a maximum capacity of 2n^2 electrons
Orbital - has a maximum capacity of 2 electrons
Give two differences between an electron in a 4s orbital and an electron in a 3p orbital
1) The electron in the 4s orbital has greater energy than the electron in the 3p orbital
2) The electron in the 4s orbital has a high probability of being found within a spherical shape, the electron in the 3p orbital has a high probability of being found in a dumbbell shape
Distinguish between a 2p orbital and a 2p sublevel
2p orbital - has a maximum capacity of 2 electrons
2p sublevel – has a maximum capacity of 6 electrons, consists of three 2 p orbitals of equal energy
How are electrons in different p-orbitals in the same p-sublevel a) the same b) different?
a) They have the same amount of energy and a high probability of being found in the same dumbbell shape
b) They have different orientation in space i.e. they lie on different axes
Aufbau Principle
Electrons must fill the lowest available sub-levels first
Why do electrons fill the 4s sublevel before the 3d sublevel?
• The 4s sublevel is lower in energy than the 3d sublevel
• Electrons fill the lowest available sub-levels first
Hund’s Rule of maximum multiplicity
When two or more orbitals of equal energy are available the electrons must fill the orbitals singly before filling them in pairs
Pauli’s Exclusion Principle
two electrons in an orbital must spin in opposite direction
How is a transition element identified from its s,p configuration?
Transition elements form at least one ion that has an incomplete 3d sublevel
Important exceptions: There are two transition elements whose s, p….. configurations are different to what is expected; copper and chromium
1. Copper
Notice: For copper, an electron moves from the 4s sublevel to the 3d sublevel. Gives the copper atom a full outer sublevel of electrons. The atom has increased stability in this state
2. Chromium
Notice: For chromium, an electron moves from the 4s sublevel to the 3d sublevel. Gives the chromium atom a half-full outer sublevel. The atom has increased stability in this state
Writing electronic/s,p….. configurations for ions
- For ions, care needs to be taken: positive ions have lost electron(s) (cations), negative ions have gained electron(s)(anions)
- Square brackets around the electron configuration with the ion’s charge are essential