CEM 142 Exam 2
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
78 Terms
what needs to happen to conduct electricity
moving charges
describe chemical reactions and what happens to define it as a chemical reaction
the chemical formula changes
at the molecular level
some bonds break and some form
the overall chemical identity of the substances changes
it involves the rearrangement of atoms
atoms are conserved
same # of atoms in the reactants as the products, they’re just rearranged
the connections (bonds) between the atoms change
break or form
reactions involve changes in location of valence electrons
reactions and reactivity can be predicted by understanding how the electrons are distributed in the reactants
two types:
acid-base
redox
describe a phase change
the formula remains the SAME
IMFs are either formed or broken/overcome
the formula/element is the same in all phases
interactions/IMFs are what changes
describe a solution process
dissolving/making solutions can sometimes be a chemical reaction
sugar/salt is just a simple solution process
same structures and formulas
no breaking/forming new ions
both states have ions, they just aren’t touching each other when in water
HCL dissolving is a chemical reaction
the products are different formulas than the reactants
the formation of a solution is sometimes a chemical reaction
important to note about H+
it’s a proton
removed an electron
there’s no such thing as H+ by itself in an aqueous solution
H+ is a very small, highly charged species
it’s always surrounded by several water molecules (a solvation shell)
the hydronium ion(H3O+) is a better way to represent H+
if you see H+ assume it’s H3O+
describe an Arrhenius acid
dissolves in water to give H+ ( a proton)
a bond was broken to get to H+
since we produced H+, that tells us that we started with an acid
describe an arrhenius base
dissolves in water to give -OH (hydroxide)
a bond was broken to give -OH
describe the arrhenius acid-base model
the simplest model
has limited applications due to its simplicity
this model ignores the role of water
anytime an arrhenius acid reacts with an arrhenius base, some kind of salt + water is produced
what is the detailed ionic equation and when would we use it mose?
shows the details
shows what ions are present (includes all the molecules)
shows what species are actually present in solution
used for arrhenius model mainly
what are spectator ions
species that don’t change during the chemical reaction
think of the detailed ionic equation
since they don’t change, they don’t take part in the reaction
what is the net ionic equation and when do we mainly use it?
still shows ions, but not all of them
just the overall
shows only the reaction taking place
used mainly with arrhenius model
formal charge equation reminder
# of valence electrons (on neutral atom) - # of bonds (on atom we’re looking at) - 3 of lone electrons (acutal # of dots, not pairs)
describe bronsted-lowry acids
a proton (H+) donor
describe bronsted-lowry bases
a proton (H+) acceptor
what are conjugate acids and bases
every conjugate acid has a conjugate base and every base has a conjugate acid
if you’re asking for a conjugate acid, that means the element is a base, basses accept protons
if you’re asking for a conjugate base that means the element is an acid, acids donate a proton
“conjugates” since the equilibrium arrow means the reaction can go in either direction
there will always be conjugate acids/bases since equilibrium and reaction arrows are used interchangeably and technically every reaction is irreversible and therefore in equilibrium, it just might be completely to one side
how does an acid become a conjugate base (bronsted)
the acid donates a proton, what remains of the acid is called the conjugate base
how does a base become a conjugate base (bronsted)
the base accepts a proton to become the conjugate acid
what’s left after the base donates a proton is called the conjugate acid
how do acids and bases react (bronsted)? example: HCL and H2O
the opposite partial charges of HCL and H2O attract
the proton that gets transferred is ‘assisted’ by the lone pair on the O
the H+ doesn’t just fall off the Cl and then hop onto the water
in order for H+ to be transferred the bond between HCL must break and a new bond between the H+ and the O forms
the proton transfers when the molecules collide
collisions is what makes this happen
molecules collide in a certain way that make it so the partial + (aka H) is attracted to the partial -
opposite charges attract which causes the molecules to collide
the acid donates a proton to the base
one bond breaks and a new one forms
during collision a proton is transferred from the acid to the base
describe the bronsted-lowry acids and bases model
more general, a broader model
a more useful definition
allows more molecules to be bases, they aren’t limited to hydroxide (-OH)
good for reactions where protons are transferred and where water is the solvent… but it’s still limited
describe how water can be an acid or a base
it’s amphiprotic
can be both an acid or base
water can react with water
it could go in both directions
if there’s given the same amount of products/reactants, there will be more neutral water molecules than ions in water
some ions are present but not that many
ions do exist since H2O molecules can react with each other
evidence: the light board in class
the lights didn’t light up so there was no electricity and not enough moving ions to conduct electricity
describe lewis acids
electron pair acceptors
have a place to accept the electrons
this doesn’t have to be a proton (as an acceptor) but can be
all bronsted acids are lewis acids
species with an empty or partially empty orbital (of available energy) are also lewis acids
orbitals that can accept electrons
group/column 3 and most transition metals
electron sink- accepts the electrons
describe lewis bases
electron pair donors
have available pair of electrons to donate into a bond (formed with the lewis acid)
to have a lewis base you need to have a lone pair of electrons
electron source
initiator of the reaction
describe the lewis acid and base model (example: HCl + H2O ←→ H3O+ + Cl-)
think of acid-base reactions as electron pair donations
this model encompasses and explains the Bronsted-Lowry and the Arrhenius theory
BUT we consider the base the electron source and the initiator of the reaction
this is an even broader model that incorporates reactions where there is no proton transfer (like group 3 with empty p orbitals)
we use arrow pushing for lewis
the base donates a lone pair to an H (most times) to form a new bond
example with HCl + H2O ←→ H3O+ + Cl-:
HCl is the acid and H2O is the base
2 electrons on the base (water) will be donated to the acid, when this happens a bond will form
the base initiates the reaction by donating a pair of electrons
the lone pair on the base turns from a lone pair of electrons to a bonding pair of electrons between the O and the new H
since the H can’t have 2 bonds, it transfers its bonding pair of electrons to the Cl to become a lone pair
the acid accepts the electron pair
what’s the difference between bronsted and lewis (specifically in terms of drawings)
bronsted:
deals with attractions and proton transfer
in the drawing include dashed line between the partially negative and positive atoms to show an electrostatic attraction
dashed line between the hydrogen to a lone pair
lewis:
deals with electron transfer
lone pair of electrons is donated to the H to form a new bond
base initiates reaction
use arrow pushing
1st arrow comes from the lone pair on the base and goes to the H on the acid
2nd arrow comes from the bonding pair on the H to the acid to make the conjugate
how can BH3 act as an acid when NH3 is the base
base initiates reaction
B has 2p2 hybridization
there’s an empty p orbital
the empty p orbital allows for 4 bonds, so none will have to break for this reaction
all of the old bonds remain in tact
1st arrow comes from the lone pair on the N and go to the Br (this is since H is similar to C in terms of electronegativity and so the Hs actually have a partial negative charge and the Br has a positive)
there is no second arrow
product: the n is bonded to the br as one big molecule and the br has a negative formal charge and the N a positive 1 formal charge
most transition metals and column 3 have an empty orbital that allow for this
how to spot an acid
all have Hs attached to an electronegative atom
acidic Hs are bonded to electronegative elements
like O, Cl, Br, I and sometimes N, or F
the H will have a partial positive charge since it’s bonded to an electronegative atom
the H-X bond is weakened by interactions with the solvent
once the acid is in a solution, the partial positive H is going to be attracted to the solvent due to the partial charge created from the electronegative atom
this attraction is what’ll weaken the bond between X-H
the conjugate base, X-, is stable
since X is electronegative, it doesn’t mind having the extra lone pair or electrons and doesn’t mind the negative charge, it can stabilize since it’s electronegative
note: if X is already negative and has an extra set of lone pairs, it doesn’t want another, even if it’s an electronegative atom. it would make the molecule more unstable
comparing acids
acids want to accept electrons/give up H+
the more electronegative an atom, the bigger the partial charge on the (most times) H bonded to it. the Hs are more attracted to the solvent (usually water) which weakens the H-X (acid) bond and it’ll be easier to break
the weakest acids have the strongest conjugate bases and also the least stable conjugate bases
the strongest acids have the weakest conjugate bases as they’re the most stable
they can better stabilize the negative charge due to a stronger electronegativity
how to spot a base
all have a lone pair of electrons that are available to form a new bond
bases HAVE to have a lone pair of electrons
this lone pair is the lone pair that’s donated in order to form a new bond
they should be (relatively more) stable when the new bond forms
the stronger the base, the weaker the conjugate acid and the more stable the conjugate acid is
typically, electronegative atoms have lone pairs… but electronegative atoms like to hold onto their electrons
so we’re looking for a perfect balance
has a lone pair but not holding on too tightly (like N)
bases donate a pair of electrons to becom a bonding pair
why don’t we include CH4 or Ne in acid/base comparisons
CH4
is not an acid
no partial charge, the C and H have almost the same electronegativity
Ne
noble gas, can’t form bonds
can’t act as a base even though it has all those lone pairs
also, it’s very electronegative and unwilling to donate electrons to form a bond anyways
why do acid base reactions occur?
they begin with the partially negative end of one molecule interacting with the partially positive end of the other and they eventually collide
we can show the reaction mechanism using curved arrows (lewis)
what are electrophiles and nucleophiles an extension of
of lewis theory
type of acid-base ration
the partial positive isn’t on the Hydrogen… it’s on a CARBON
describe electrophile
a lewis acid
electron loving or negative charge loving
when the partial positive part of a molecule is a Carbon, we call the molecule an electrophile
has to be a partial Positive charge so it’ll be attracted to the electrons
accept electrons
the positive has to be on the Carbon, the carbon is the electrophilic site
describe nucleophile
a lewis base
nucelus loving or positive charge loving
an electrophile reacts with a nucleophile
donates electrons
describe CH3Br (electrophile) reacting with NH3 (nucleophile)
*disregard light-colored arrow on bottom left
when the partial positive isn’t on a Hydrogen
this is called methylation
added a methyl group to a N
CH3 = methyl group
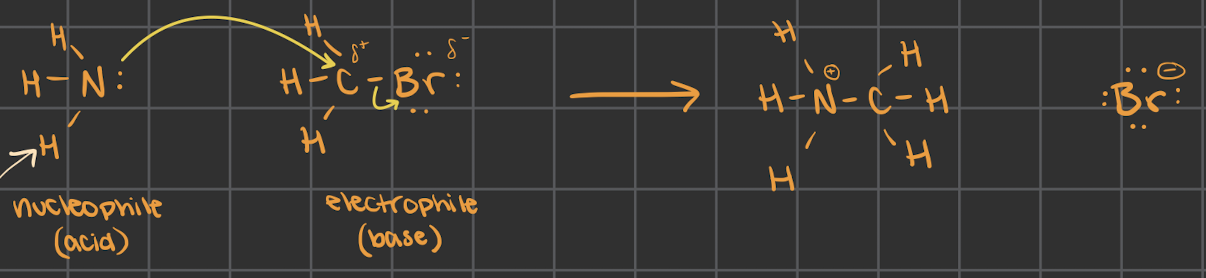
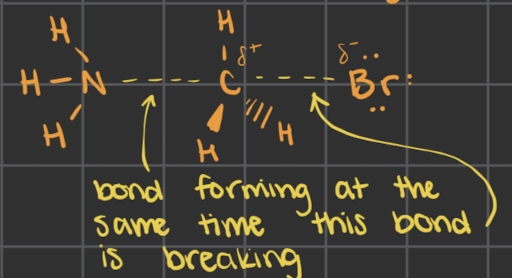
what is the transition state (use CH3Br and NH3 as an example)
we have both things happening at the same time
as one bond forms, the other one breaks
the thing that exists as we transition from the reactants into the products
this is what the reactants go through as they transition from reactants to products
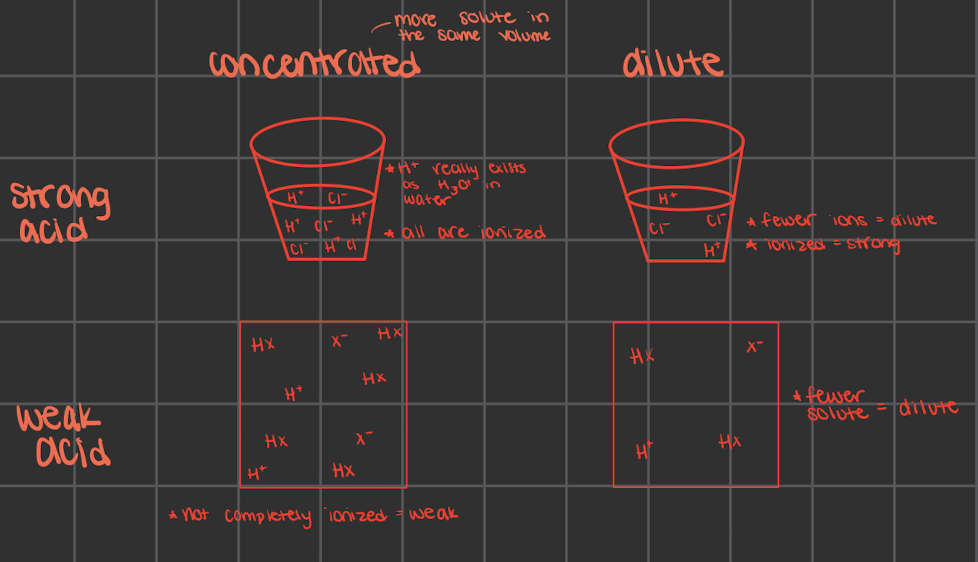
what does strong mean for acids/bases
strong acids are fully ionized in a solution
every molecule breaks into their separate ions
strong acids would make a light bulb glow more since there would be more ions to have around as charges
evidence: using the light with the circuits (in class) and compare brightness
no equilibrium arrow really since it fully ionizes
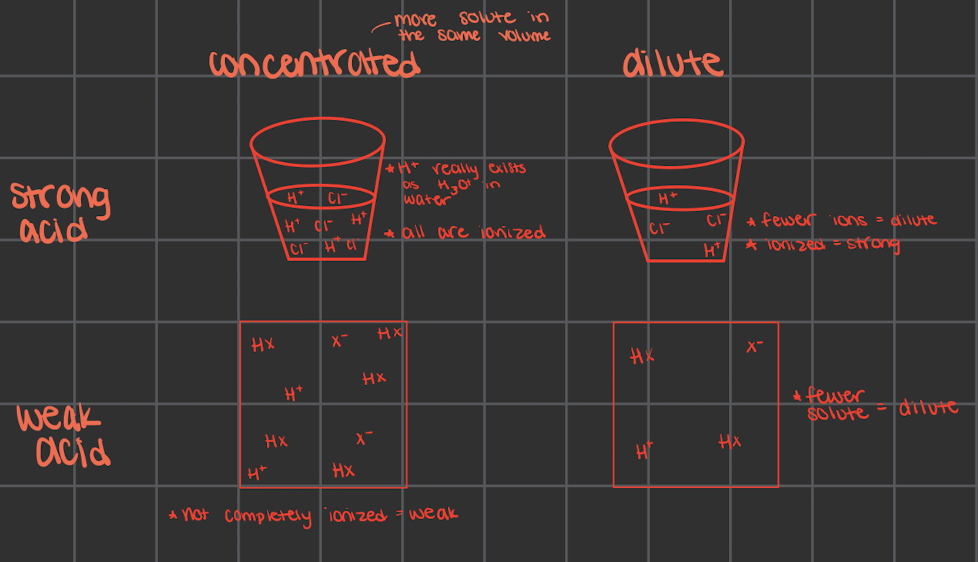
what does weak mean for acids/bases
not fully ionized in solution
only some molecules break into ions
only partially ionize in solution which causes the light bulb to glow relatively dimly at the same concentration
there is more of the molecular form than the ionized form
there are still equilibrium arrows but ones longer and ones shorted
longer: more of whatever it’s pointing towards
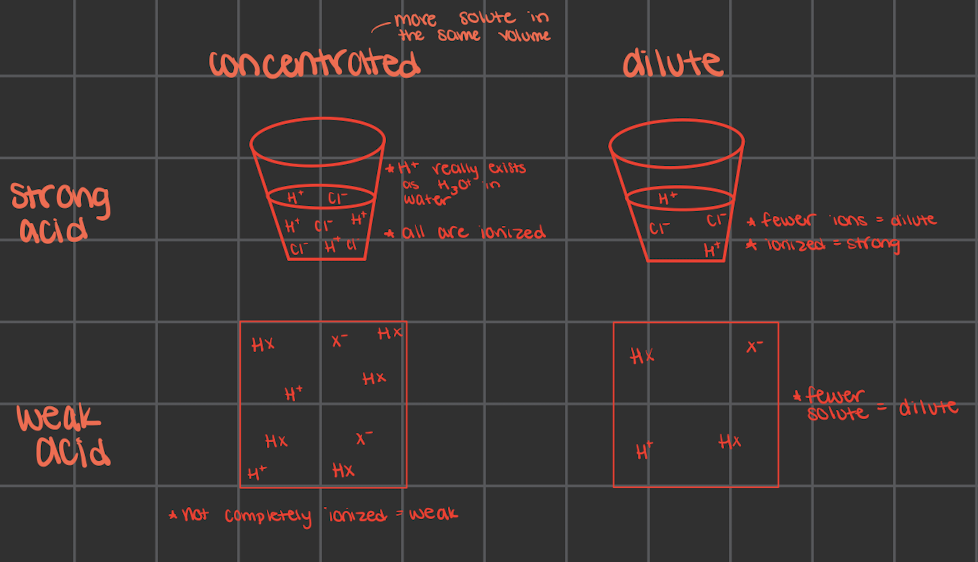
dilute vs concentrated
only tell about the amount of acid/base, not about the strength
could have a concentrated/dilute strong/weak acid/base
strong and weak doesn’t always matter if there’s more solute (aka concentrated) there’s more ions that can move
describe acid strength across a row
increases across a row (left → right)
due to electronegativity the electronegativity increasing across a row
a F-H (in row 2 for example) is the most polarized so the H will have a greater partial positive charge and therefore H is more likely to attract a base by interacting with its lone pair
as the acid goes into solution (like H2O as the base) there’s a STRONG interaction between the acidic H and the O of the water
this is what weakens the F-H bond
the F- conjugate base is the most stable due to F’s high electronegativity. The extra pair of electrons is attracted closer to the nucleus and is therefore more stable
the conjugate base is stable (it can hold the negative charge) which means that electronegative atoms are most likely to be bonded to acidic Hs
the more electronegative the atom, the less likely it’ll donate/share it’s electrons and it’ll be attracted to more electrons
what type of evidence would you use for describing acid strength across a row in terms of HF for an example
the F is the most electronegative (compared to the other atoms in the row) so H will have the biggest partial positive charge and will interact the most strongly with the solvent/base
OR
F- is the most stable conjugate base
meaning it’s the weakest base and tied to the strongest acid
describe acid strength down a group and what it depends on
cannot use electronegativity to explain acid strength down a group
think thermodynamics
strong acids completely ionize (super - ∆G)
weak acids partially ionize (kinda - ∆G)
the stronger the acid (and the more it ionizes) the farther right the reaction will go
the more thermodynamically favorable a process is, the more - ∆G
acid strength depends on:
the enthalpy change ∆H when added to water
the strength of the H-X bond (that you’re breaking and forming)
the stability of the X- conjugate base
the entropy change ∆S when added to water
the number of arrangements (before and after the reaction) for the ions AND water molecules
∆s and ∆H contribute to ∆G
what impacts enthalpy ∆H when comparing acid strength down a row but we don’t focus on? and why don’t we focus on it?
when an acid (HX) is placed in water and it dissolves and ionizes
when it dissolves:
it’s a solution process
IMFs are overcome in the solute and solvent
solute-solute and solvent-solvent interactions are overcome
requiring energy
solute-solvent interactions form
releasing energy
∆H from these 2 process is relatively small since IMFs are weak, so we ignore it in our analysis but it still exists/happens
what impacts enthalpy ∆H for acid strength down a column
when HX ionizes
HX bonds break
requires energy (+∆H)
not the same for all the acids
they all have different bond strengths
O-H bonds form
releases energy (-∆H)
when H2O is the solvent
an O-H bond will be a certain strength no matter what the acid is
∆H from these 2 processes is relatively large (bonds are stronger than IMFs)
the energy released upon forming OH bonds with water will be the same no matter what the acid is
the energy absorbed upon breaking and HX bond will depend on the strength of that bond
bond enthalpy: the energy it takes to break the HX bond in the gas phase (∆H = +)
the more overlap between orbitals (aka the more similar size orbitals and therefore atoms) the stronger the bond is
a bigger atom, meaning a smaller % of the orbital is overlapping, the weaker the bond
this means it requires less energy to break HI than HF (for example) and ∆H is the least positive for HI
forming an OH bond releases energy and this energy is more than it takes to break the HX bond
more energy is released (from the OH bond) than absorbed (from the HX bond) overall so ∆H will be negative for all, it’s a matter of how negative
the most -∆H contributes to a more -∆G
∆H = OH + HX
OH: always the same and very negative since it releases energy
depends on acid and always + since it breaks and requires energy
what impacts entropy ∆S for acid strength down a group
∆G = ∆H - T∆S
subtracting a (-) means adding a number
subtracting a (+) means minusing a number
a more (+) ∆S will contribute to a more -∆G
determined by ion sizes
example comparing F- and I-
they both have a -1 charge but the (-) chare is more spread out over different-sized atoms
the charge is more concentrated in F than in I which causes stronger interactions between F- and H2O
water can’t have as many arrangements since more are ‘locked’ in around F than I
smaller ions (like F) attract water molecules tightly which limits the water molecules’ positions (so entropy decreases -∆S)
large ions don’t attract water molecules as tightly so the water molecules can occupy more positions (so a more +∆S as the entropy increases)
put together the ideas of enthalpy and entropy to describe acid strength down a column
the more - ∆G the more the reaction lies to the right and the more the molecules break into ions so the stronger the acid
acid strength increases down a column
due to enthalpic and entropic effects
cannot use electronegativity
Enthalpic Effect
the bond strength HX decreases down a group (weaker bonds) due to the difference in size of H and X
the difference in size between H and X gets bigger down a group
there’s less overlap between H and X orbitals when they’re different sizes
The HX bond is weaker and easier to break
takes/requires less energy
Entropic Effect
entropy decreases when water molecules solvate small, highly charged ions
water molecules get locked in place and have fewer arrangements
determine acid strength if H is bonded to the same type of atom in different molecules
reminder:
the strongest acid has the weakest and most stable conjugate base
it’s stable because it’s weak and doesn’t need to react
the strongest acid will have resonance structures
the lone pair on the resonance could become a bonding pair but the C can’t have 5 bonds so one of the other bonds becomes a lone pair on the other Oxygen
the negative charge can be considered to be ½ on each oxygen
a resonance hybrid
this better stabilizes the negative charge since it’s spread out over more atoms
which means the conjugate base is the most stable → weaker conjugate base → strongest acid
**induction effect may work for other types of examples too
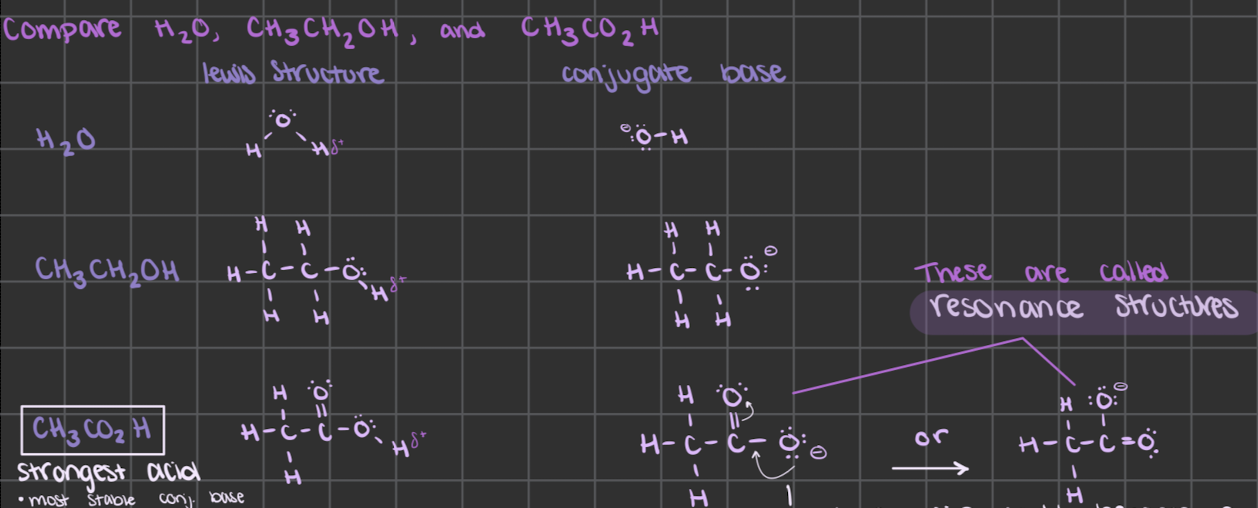
describe how strong acids have weak conjugate bases
the stronger the acid, the weaker its conjugate base
conjugate bases can be stabilized (made weaker) by spreading out the negative charge over several atoms
through resonance structures
compare -OH to H2O for base strength
-OH is a stronger base
the negative on the -OH means it has extra electrons and therefore more willing to donate a pair of electrons (lewis model)
compare NH3 to H2O for base strength
NH3 is a stronger base
O and H are in the same row so you can use electronegativity
the N is less electronegative than O so it’s not pulling on the lone pair of electrons as hard and is more willing to donate/share the lone paire
likewise: the O is more electronegative and is pulling on the lone pair more so it’s less willing to share
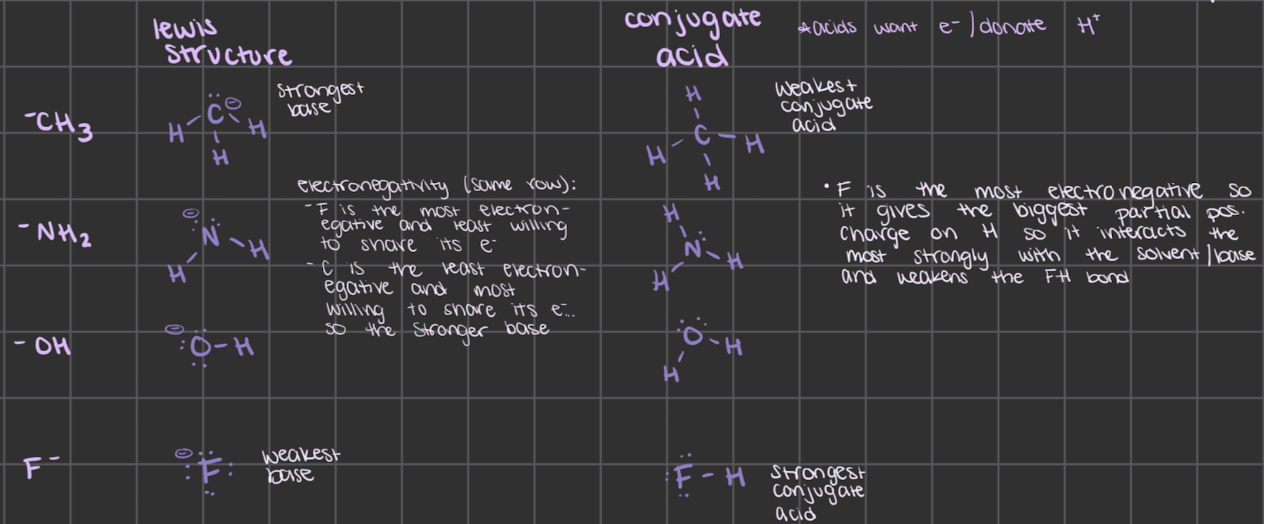
which is the stronger base when comparing -CH3 to -NH2 to -OH to F- and why
use lewis structures
use electronegativity since they’re the same row
F is the most electronegative and least willing to share its electrons
C is the least electronegative and most willing to share its electrons so it’s the stronger base
OR conjugate acids
F is the most electronegative so it gives the biggest partial positive charge on H so it interacts the most strongly with the solvent/base and weakens the FH bond
how to determine the direction of acid-base reactions when at an equilibrium
stronger acid + stronger base ← ———> waker acid + weaker base
there will always be more of the weaker acid and base
the reaction can go both ways (hence equilibrium arrows)
but it doesn’t go in both directions equally
we say that the equilibrium lies to one side or the other
more reactants or products are present at equilibrium
the stronger acid and base are always on one side together and the weaker acid and base are on the other
list of strong acids
assume weak acid for anything else unless specified
*completely ionizes
HCL
HBr
HI
HNO3
H2SO4
list of strong bases
*completely ionizes
group 1 or 2 with hydroxide (OH)
example: NaOH
what’s water’s concentration
55.5 M
how to find % ionization
% ionized = (ionized/total) x 100
total could be found by ionized + ionized or by how much acid/base you started with/were given
how to find pH and how to find [H3O+]
pH = -log[H3O+]
log base 10
note: the pH can go beyond 1-14 at both ends
[H3O+] = 10-pH
what is pH
tells the concentration of H+ (but really H3O+) in solution
this is since H+ doesn’t exist by itself in the solution
pH tells how acidic or basic a solution is
high pH: more basic
less [H3O+]
more [-OH]
low pH: more acidic
more [H3O+]
less [-OH]
what is auto-ionization of water and what does this tell us about the [H3O+] and the [-OH]
when water reacts with itself
this is rapid and reversible in water:
there are more neutral water molecules than ions
evidence: light board in class
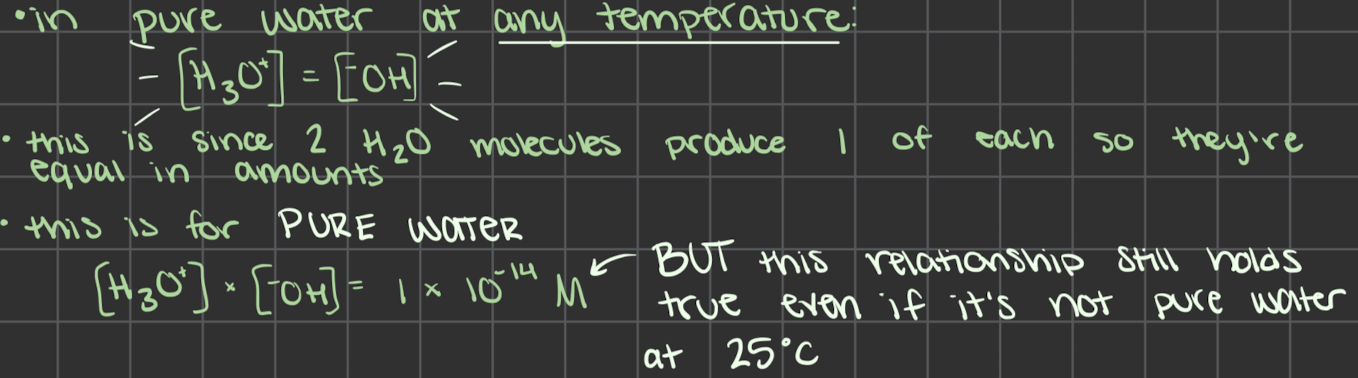
in pure water the concentration of H3O+ equals the concentration of -OH
[ ] = concentration (M)
at 25˚C the concentration of each ion is 1 × 10-7M
[H3O+] = [-OH] = 1 × 10-7M
how to find [H3O+] [-OH] from each other at 25˚C
[H3O+] x [-OH] = 1 × 10-14
at 25˚C
[H3O+] = 1 × 10-14 / [-OH]
[-OH] = 1 × 10-14 / [H3O+]
what does pH depend on
TEMPERATURE
this solution (in the pic) is neutral:
the concentration of H3O+ and -OH are equal so the solution is neutral
the solution is pure water
pH depends on temperature
acidic vs basic vs neutral
deals with relative concentrations of H3O+ and -OH
acid = more H3O+
base = more -OH
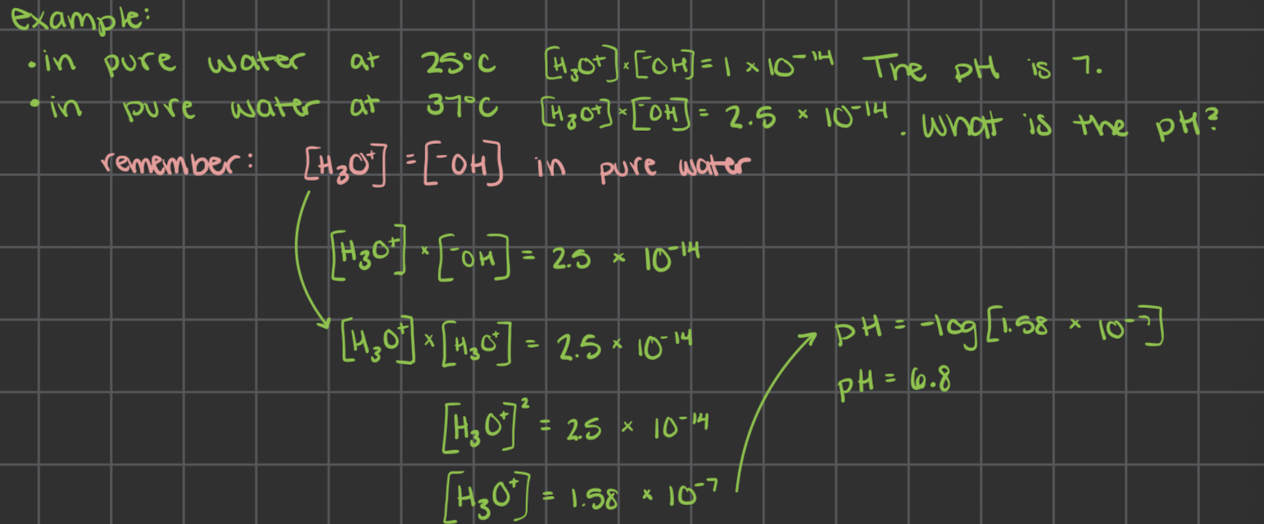
[H3O+] and [-OH] relationship
how to find [H3O+] when given a strong acid or how to find [-OH] when given a strong base
*the same holds true from the image for strong bases
strong bases fully ionize
not all strong bases have a super high pH
likewise with acids at low pHs
pH depends on [H3O+] and [-OH], not necessarily strong vs weak
![<p>*the same holds true from the image for strong bases</p><ul><li><p>strong bases fully ionize</p><ul><li><p>not all strong bases have a super high pH</p><ul><li><p>likewise with acids at low pHs</p></li></ul></li><li><p>pH depends on [H<sub>3</sub>O<sup>+</sup>] and [<sup>-</sup>OH], not necessarily strong vs weak</p></li></ul></li></ul>](https://knowt-user-attachments.s3.amazonaws.com/5c8b3696-428b-4ce2-a84c-51de46b1f40b.jpeg)
describe redox reactions and oxidation vs reduction
oxidation-reduction reactions
both oxidation and reduction are happening in the reaction
one thing is being oxidized and another is reduced
oxidation
if an atom loses one or more electrons it’s being oxidized
lose valence electrons
reduction
gaining 1 or more electrons
reducing in charge
*the electrons from what’s being oxidized are transported/going to what’s being reduced
OLI RIG
Oxidation Is Loss (of electrons)
Reduction Is Gain (of electrons)
what are oxidation state/numbers
used to keep track of where electrons are before and after reaction
do not confuse with formal charges
both keep track of electrons but tell you different things
they’re a way of electron book-keeping
allows you to see whether the element has lost or gained electrons (or a bigger share of the electrons) during the reaction
if oxidation #s change during a reaction then it’s a redox reaction
if they don’t, then it’s not a redox
common rules for oxidation numbers
atoms in pure elements = 0
examples: H2, O2, Na
ions = ion charge
example: NaCl
Na = +1
Cl = -1
total/sum = 0
oxygen = -2
hydrogen = 1
the sum of a molecule’s oxidation #’s should equal 0
unless there’s a formal charge on the molecule, then the sum of the oxidation #’s should equal the charge
trick for assigning oxidation #s
withdraw all of the electrons in each bond to the most electronegative atom
remember that this doesn’t actually happen
once you pull the electrons to the more electronegative atom you calculate formal charge (to find the oxidation number)
formal charge vs. oxidation numbers
**both are ways of electron book-keeping
formal charge
treating bonding electrons like one electron belongs to one atom and one electron belongs to the other
used to draw the most stable lewis structures
better to have fewer and lower # formal charges in lewis structures
oxidation number
treating the bonding electrons like they both belong to the more electronegative atom
used to determine if a reaction is a redox reaction or not
keeps track of electrons and where they’re transferred in a redox reaction
we ‘give’ all the electrons to the most electronegative atom in a bond
they decide if a redox reaction happens or not
what are resonance structures
if you can take 1 structure and use curved arrows to get to the other structure, that’s how you know you have resonance
since the charge is spread out/split between atoms, it can cause the acid to be stronger since it stabilizes the negative charge better on the conjugate base, making it a weaker conjugate base
also, the spread out negative causes a weaker conjugate base since the lower magnitude of charge won’t attract a proton as easily
a resonance hybrid
this better stabilizes the negative charge since it’s spread out over more atoms
which means the conjugate base is the most stable → weaker conjugate base → strongest acid
why do we use multiple models of acid-base reactions?
many models help us predict and explain outcomes. generally, we use the simplest model that’ll work in a given solution. even though all acid-base reactions can be described using the lewis model, sometimes it is simpler and easier to use the bronsted-lowry model. this is partially true for reactions in aqueous solution where proton transfer is the easiest way to think about the reaction
2 ways of explaining stronger acids using the bronsted model
discuss electronegativity and how H has a bigger partial positive so it’s more likely to be given away and is more attracted to the base/solvent
electronegativity: after it donates the proton, it can better stabilize the negative charge
the stronger the acid, the weaker the conjugate base and more stable the conjugate base
thermodynamics (down a column for acids)
bronsted to explain stronger bases
bases have to accept protons
the atom has room for another bond (aka a lone pair) and the atom has a partial negative charge which will attract the proton
lewis model to explain stronger acids
acid accepts a lone pair from the base
the more electronegative atom will have stronger interactions with its lone pair so it’s less likely to give up its electrons
lewis model to explain stronger bases
bases are electron pair donors
the less electronegative atom is more likely to give up its electron pairs
describe the beginning, middle, and end of reactions
beginning
acid-base are interacting (usually through hydrogen bonding interactions)
middle/transition state
the bond forming and breaking happens at the same time
the HX (acid) bond breaks as the HY (base) bond forms
the H doesn’t fall off and reattach
the H+ bonded to X is attracted to the lone pair on Y and the HX bond is weakened until it’s overcome
end
they’re ionized
what happens to the H3O+ if we add solute to a solution and the pH stays the same
the H3O+ stays the same too
the solute didn’t act as an acid or a base
the solute doesn’t react with the water/solvent
strong vs weak acid/base using concentrations
if the given is strong (acid or base) then the H3O+ or -OH concentration will equal the amount of the given (acid or base)
then you can solve for pH
the pH is what the pH should be if it were a strong acid or base
you can compare when the concentration of the substances are the same
Why is CN- not an acid?
it’s already negative
more electrons would make it unstable
wants to get rid of those extra electrons
what are inductive effects
when electronegative atoms in a molecule pull the other electrons (electron density) toward themselves
for a base (for example) to be more stable, the charge on the base is better stabilized since it’s more spread out
the electronegative atoms pull the electron density toward themselves making the base less likely to share its electrons
the less spread out the charge = the more basic