W15: CARBOXYLIC ACIDS
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
A ____________ is an organic compound whose functional group is the carboxyl group.
carboxylic acid
A ____________ is a carbonyl group (CO) with a hydroxyl group (—OH) bonded to the carbonyl carbon atom.
carboxyl group
Abbreviated linear designations for the carboxyl group are:
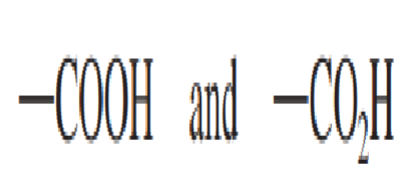
The __________________ has a hydrogen atom attached to the carboxyl group carbon atom.
simplest carboxylic acid
Draw the structure of simplest carboxylic acid:
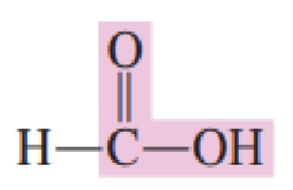
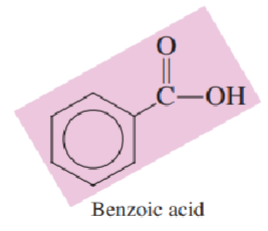
The structure of the _______________________________ involves a benzene ring to which a carboxyl group is attached.
simplest aromatic carboxylic acid
Is an organic compound that can be synthesized from or converted into a carboxylic acid.
Carboxylic derivatives
Four important families of carboxylic acid derivatives are:
1.) Esters
2.) Acid chlorides
3.) Acid Anhydrides
4.) Amides
Draw structure of Ester:
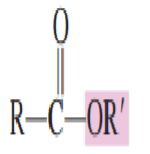
Draw the structure of Acid chloride
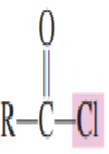
Draw the structure of acid anhydride
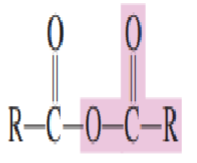
Draw the structure of Amide:
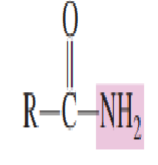
A __________________ is a carboxylic acid in which one carboxyl group is present.
monocarboxylic acid
In naming monocarboxylic acid, name the parent chain by changing the -e
ending of the corresponding alkane to __________.
-oic acid
What are the three simplest carboxylic acids?
1.) Methanoic acid
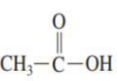
2.) athanoic acid
3.) propanoic acid
Draw the structure of Methanoic acid:
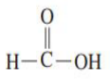
Draw the structure of Ethanoic acid:
Draw the structure of Propanoic acid:
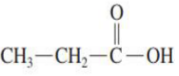
Is a carboxylic acid that contains two carboxyl groups, one at each end of a carbon chain.
Dicarboxylic acids
The simplest aromatic carboxylic acid is called __________.
benzoic acid
Draw the structure of benzoic acid:
It is the stinging sensation associated with red ant bites.
Formic acid
Vinegar contains small amount of _____ acid
acetic
Is the smallest acid that can be obtained from fats
Propionic acid
Component of a rancid butter.
Butyric acid
Found in valerian foot, it has a strong odor.
Valeric acid
Skin secretions of goat
Caproic acid
It is the “most” widely used of all carboxylic acids.
Acetic acid
It is the simplest carboxylic acid, found in plants.
Oxalic acid
Play important roles in biochemical reactions that occur in the human body.
Succinic and Glutaric acid
Is a carboxylic acid that contains one or more additional functional groups besides one or more carboxyl groups.
Polyfunctional carboxylic acids
Three commonly encountered types of polyfunctional carboxylic acids are:
1.) Unsaturated acids
2.) Hydroxy acids
3.) Keto acids
Draw the structure of an unsaturated acid:
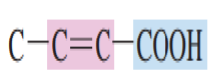
Draw the structure of hydroxy acid:
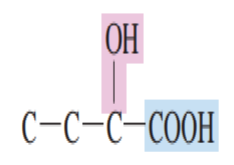
Draw the structure of a keto acid:
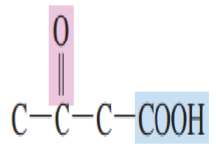
This is the simplest unsaturated mono carboxylic acid.
Propenoic acid
Unsaturated acid trans isomer
Fumaric acid
Unsaturated acid Cis isomer
Maleic acid
Four simpler hydroxy acids are:
1.) Glycolic acid
2.) Lactic acid
3.) Malic acid
4.) Tartaric acid
Is present in the juice from sugar cane and sugar beets
Glycolic acid
Is present in sour milk, sauerkraut, and dill pickles.
Lactic acid
Occurs naturally in fruits.
Malic acid and Tartaric acid
Sharp taste of the apple.
Malic acid
Abundant in grapes, component of tartar sauce, and acidic ingredients in many baling ingredients.
Tartaric acid
The best known of all carboxylic acids. Gives citrus fruits their sharp
taste, widely used in beverages and foods. Tricarboxylic acid.
Citric acid
As the designation implies, contain a carbonyl group within a carbon chain.
Keto acid
With three carbon atoms, is the simplest keto acid that can exist.
Pyruvic acid
Carboxylic acids are the most ___________ compounds
polar organic
Both the carbonyl part and the hydroxyl of the carboxyl functional group are ________.
polar
The result is very ____________________ for carboxylic acids, the highest of any type of organic compound yet considered.
high melting and boiling points
Unsubstituted saturated monocarboxylic acids containing up to _______________ are liquids that have strong, sharp odors
nine carbon atoms
Acids with ____________________ in an unbranched chain are waxy solids that are odorless (because of low volatility).
10 or more carbon atoms
Aromatic carboxylic acids, as well as dicarboxylic acids, are also ________________________.
odorless solids
The high boiling points of carboxylic acids indicate the presence of ____________________________________.
strong intermolecular attractive forces
Carboxylic acids readily hydrogen-bond to _____________.
water molecules
Such hydrogen bonding contributes to water solubility for ____________ carboxylic acids.
short-chain
Oxidation of _____________ or __________, using an oxidizing agent such as ________ or ___________, produces carboxylic acids.
1.) primary alcohols
2.) aldehydes
3.) CrO3
4.) K2Cr2O7
Aromatic acids can be prepared by oxidizing a _______________ on a _____________.
1.) carbon side chain
2.) benzene derivative
Is a carboxylic acid derivative in which the —OH portion of the carboxyl group, has been replaced with an —OR group.
Ester
In linear form, the ester functional group can be represented as:
COOR or -CO2R