Ch 4.5-4.7 : Redox (Oxidation-Reduction) Reactions and Equilibrium
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
____ gains electrons and is reduced.
oxidizing agent
____ loses electrons and is oxidized.
reducing agent
For an atom in its elemental form, the ON number equals __.
0
For a monatomic ion, the ON equals __.
the ion charge.
The ON for the sum of all atoms in a compound is __.
0 (talking about a neutral compound)
The ON for the sum of all atoms in a polyatomic ion is __.
polyatomic ion’s charge
For group 1A elements, the ON is __.
+1
For group 2A elements, the ON is __.
2+
For group hydrogen elements, the ON is __.
O.N. = +1 in combination with nonmetals
O.N. = −1 in combination with metals and
boron (e.g. NaH)
For group fluorine elements, the ON is __.
O.N. = −1 in all compounds
For group oxygen elements, the ON is __.
O.N. = −1 in peroxides (e.g. H2O2)
O.N. = −2 in all other compounds (except with
F)
For Group 7A, the ON is __.
O.N. = −1 in combination with metals,
nonmetals (except O),
and other halogens lower in the group
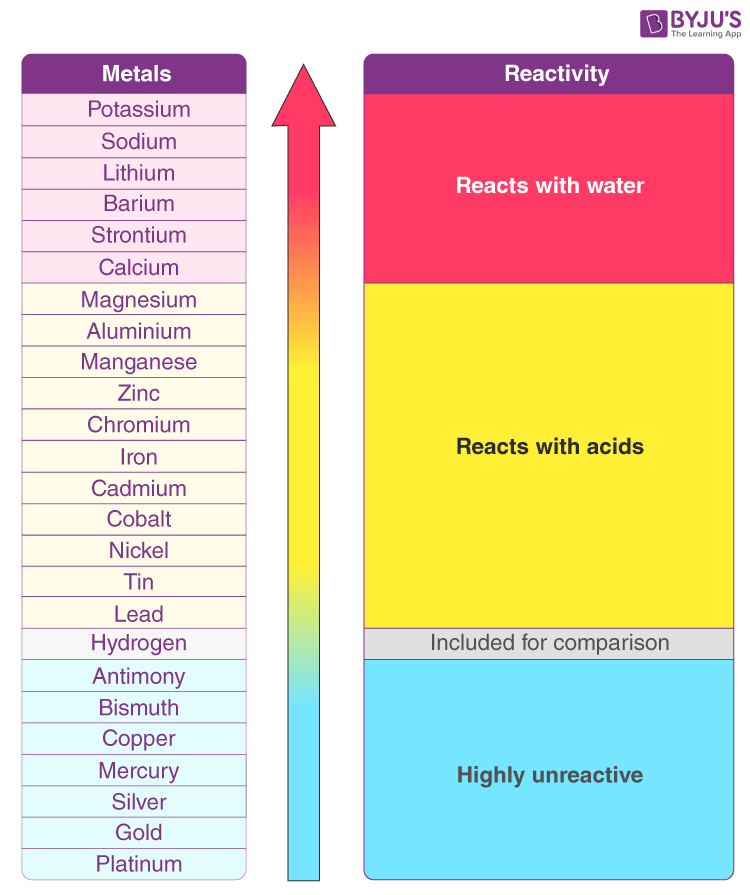
label activity series of the metals