acids bases salts
1/46
Earn XP
Description and Tags
note: for IB, use reversible arrows as long as WA/WB is involved. only use -> when it is a salt that is dissociating fully.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
acids properties
pH 1 to 7 @ 25C
releases H+ when dissolved in water
sour
reacts with bases to form salt + water
reacts with carbonates to form salt + water + CO2
reacts with metals to form salt + H2
arrhenius theory
acid: has H in formula and produces H+ ions when dissolved in water
base: has OH in its formula and produces OH- ions when dissolved in water
limitations
restricted to reactions that occur in aqueous solutions (excludes gases eg HCl + NH3 → NH4Cl)
many substances that release OH- in water do not have OH in the formula (eg ammonia, amines)
bronsted-lowry theory
acid: proton donor (ie: must have H)
base: proton acceptor (need not have H)
involves transfer of proton from acid to the base (need not be in water)
if acid reacts with alkali, it acts as bronsted-lowry acid
if acid reacts with stronger acid, it acts as bronsted-lowry base
conjugate acid-base pairs differ by a proton (H+)
acid donates proton to become conj base
base accepts proton to become conj acid
there is an acid and base on both sides of the reaction. system reaches state of equilibrium based on relative strengths of acid, base and their conjugates.
amphiprotic
species that can act as both bronsted-lowry acid and bronsted-lowry base (H+ donor/acceptor)
polyprotic
acid that has many protons (H+)
lewis theory
acid: electron-pair acceptor, to form dative covalent bond → eg AlCl3, BF3, BeCl2 have empty orbital to accommodate (BeCl2 has two)
note: not AlF3 (ionic)
base: electron-pair donor, to form dative covalent bond
product is called adduct (a species that contains a new dative covalent bond)
all atoms in the adduct have octet electron configuration
example: complex ion formation
lewis base: ligand (electron-rich, electron pair donor)
lewis acid: central metal cation (electron-deficient, electron pair acceptor)
example: NH3 + BF3/AlCl3 → H3NBF3 / H3NAlCl3
widest scope: doesn’t need to have H or OH
alkalis
bases that are soluble in water
pH>7 @ 25C
releases OH- ions when dissolved in water
bitter
reacts with acids to form salt + water
reacts with ammonium salts to give salt + water + NH3 (g)
note: this is an acidic salt, since NH4+ + conjugate acid → NH3 (conjugate base)
acid deposition
process by which acid forming pollutants (NOx, SO2) are deposited on earth’s surface
effects
deforestation
leaching of minerals from soils → increasing acidity of lakes and rivers + uptake of toxic materials from soil by plants/shellfish
corrosion of limestone buildings
pH of rainwater
naturally around 5.6
CO2 (g) + H2O (l) <=> H2CO3 (aq) ← carbonic acid (weak acid)
H2CO3 (aq) <=> H+ (aq) + HCO3- (aq)
HCO3- (aq) <=> H+ (aq) + CO3 2- (aq)
oxides of nitrogen
formation: high temperature in internal combustion engines, nitrogen and oxygen in air react to form nitrogen monoxide → which further reacts with oxygen from atmosphere to form nitrogen dioxide
N2 (g) + O2 (g) → 2NO (g)
2NO (g) + O2 (g) → 2NO2 (g)
NO2 effects
brown smog
acid rain: 2NO2 (g) + H2O (l) → HNO3 (aq) + HNO2 (aq)
then oxidised by oxygen in atm: 2HNO2 (aq) + O2 (g) → HNO3 (aq)
removing oxides of nitrogen
catalytic converter
sulfur dioxide
from volcanic eruptions and combustion of fossil fuels with sulfur impurities
formation of sulfurus acid: SO2 (g) + H2O (l) <=> H2SO3 (aq)
oxidised by oxygen in atm to form sulfur trioxide: SO2 (g) + O2 (g) <=> 2SO3 (g)
then dissolves in rainwater to form sulfuric acid: 2SO3 (g) + O2 (g) → SO4 2-
removal of sulfur dioxide
pre combustion: crush and mix coal with sulfur solvent then wash
post combustion (removing sulfur from flue gas): remove SO2 by spraying with Ca(OH)2 to form CaSO3 and H2O
soil too acidic: add CaO/CaCO3 to neutralise
ocean acidification
50% of CO2 produced by fossil fuels is dissolved by oceans → ocean becomes more acidic, inhibits shell growth + causes reproductive disorders in fish
CO2 dissolves in rainwater to form carbonic acid: CO2 (g) + H2O (l) → H2CO3 (aq)
carbonic acid forms hydrogencarbonates/carbonates
H2CO3 (aq) <=> HCO3- (aq) + H+ (aq)
H2CO3 (aq) <=> CO32- (aq) + H+ (aq)
self-dissociation of water
partial dissociation: most remains as H2O molecules → since partial, can use reversible arrow + write eq constant (Kc)
H2O (l) + H2O (l) <=> H3O+ (aq) + OH- (aq)
note: this is an acid-base reaction where one H2O is acid and the other is base
endothermic reaction
when temp increases, POE shifts right (favour endothermic) to oppose the temp increase → Kw increases as temperature increases
Kw
Kw = [H+][OH-]
Kw = Ka X Kb
pKw
pKw = pH + pOH
[H+] in strong acids
[H+] = [SA] + [H+]H2O
(if significant ie [SA] <10-7 @ 25C)
strong acids
undergo complete dissociation in water → high concentration of H3O+ ions
conjugate base is neutral since Kb << Kw
examples: HNO3, H2SO4, hydrogen halides (except HF), HClO4
acid strength of hydrogen halides increase down group 17: halogen atom radius increase, H-X bond length increase so weakened → less energy needed to break to form H+
neutral solution
[H+] = [OH-]
strong bases
undergo complete dissociation in water → high concentration of OH- ions
conjugate acid is neutral since Ka << Kw
examples: group 1/2 metal hydroxides
weak acids
undergo partial dissociation in water since low tendency to donate proton (use reversible arrow) → low concentration of H3O+ ions
definition is independent of pH (concentration), it depends on pKa (extent of dissociation)
conjugate base of a WA is a WB
the weaker the WA, the stronger its CB (but they’re all still weak) → POE lies towards the acid
weak bases
undergo partial dissociation in water since low tendency to accept proton (use reversible arrow) → low concentration of OH- ions
definition is independent of pH (concentration), it depends on pKb (extent of dissociation)
conjugate base of a WB is a WA
the weaker the WB, the stronger its CA (but they’re all still weak) → POE lies towards the base
experimental determination of acid/base strengths
enthalpy change of neutralisation is less exothermic for WA-SB or SA-WB reactions
WA/WB only partially dissociates → less energy needed to break bonds
electrical conductivity lower for WA/WB
WA/WB only partially dissociates → lower concentration of mobile ions
rate of reaction lower for WA/WB
WA/WB only partially dissociates → concentration of H+/OH- lower → rate = k[H+][OH-]
pH: at same temperature and same concentration, pH of WA higher than SA , pH of WB lower than SB (related to concentration of H+/OH- ions)
Ka
Ka = [H+]2/[HA]
acid dissociation constant: extent to which acid dissociates in water → measure of strength of acid
if ask for which eqn gives expression, chose the one involving water
the larger the Ka, the stronger the acid
the larger the pKa value, the weaker the acid
Assumes [HA] @ equilibrium = [HA] initial
Kb
Kb = [OH-]2/[B]
measure of strength of base
the larger the Kb, the stronger the base
the larger the pKb, the weaker the base
salt hydrolysis
when salt dissolved in water, undergoes further reaction with water to produce H3O+ or OH- ions, resulting in an acidic/alkaline solution.
is a reversible reaction
occurs for salts that contain
an anion that is a CB of a WA
WB + SA → acidic salt (WA) + neutral
WA + H2O <=> WB + H3O+
Ka = [WB][H+]/[acidic salt] <1
a cation that is a CA of a WB
WA + SB → basic salt (WB) + neutral
WB + H2O <=> WA + OH-
Kb = [WA][OH-]/[basic salt] <1
a cation of high charge density (Al3+, Cr3+, Fe3+)
high charge density → form coordinate bond with ligands to form complex ion
high charge density of metal cation polarises H2O → H2O pulls electrons closer to itself, O-H bond breaks
H+ released, complex ion acts as a weak acid when hydrolysed → therefore salt with a transition metal is an acidic salt.
[Al(H2O)6]3+ + H2O <=> [Al(H2O)5(OH)]2+ + H3O+
explain whether 0.1M NaNO3 solution is acidic, alkaline or neutral
NaNO3 → Na+ + NO3-
NaNO3 formed from strong acid HNO3 and strong base NaOH. neutral since Na+ and NO3- do not undergo hydrolysis.
explain whether 0.1M Na2CO3 solution is acidic, alkaline or neutral
Na2CO3 → 2Na+ + CO3 2-
Na2CO3 is formed from strong base NaOH and weak acid H2CO3
Na+ ions do not undergo hydrolysis because metal cations do not react with water
CO3 2- + H2O <=> HCO3- + OH- (WB + H2O <=> WA + OH-)
the conjugate base (CO3 2-) of the weak acid undergoes hydrolysis to form basic solution due to OH- formed
(recall if salt has anion that is CB of WA, then it undergoes hydrolysis)
buffer solutions
solutions that maintain an approximately constant pH when small amonts of either acid or base are added/when solution is diluted
has acidic component and basic component
formed by direct 1:1 mixing of WA/WB + conjugate
OR 2:1 excess weak + limited strong
how does acidic buffer work?
salt fully dissociates (→) to give large amount of conj base anions (WB) + cation from SA
WB reacts with H+ from forward reaction to produce conj acid (the WA we started with)
this backward reaction suppresses the forward dissociation reaction (of the WA)
when small amount of H+ added, CB + H+ <=> CA. added H+ is removed as CA, [H+] remains constant.
when small amount of OH- added, CA + OH- <=> CB. added OH- is removed as CB, [OH-] remains constant.
pH = pKa + lg [salt]/[acid]
pH points to consider for pH titration curves
initial pH → SA/SB/WA/WB
equivalence pH @ Veq → gradient vertical
pH = pOH
pH = 7: SA + SB
pH < 7: SA + WB
pH > 7: WA + SB
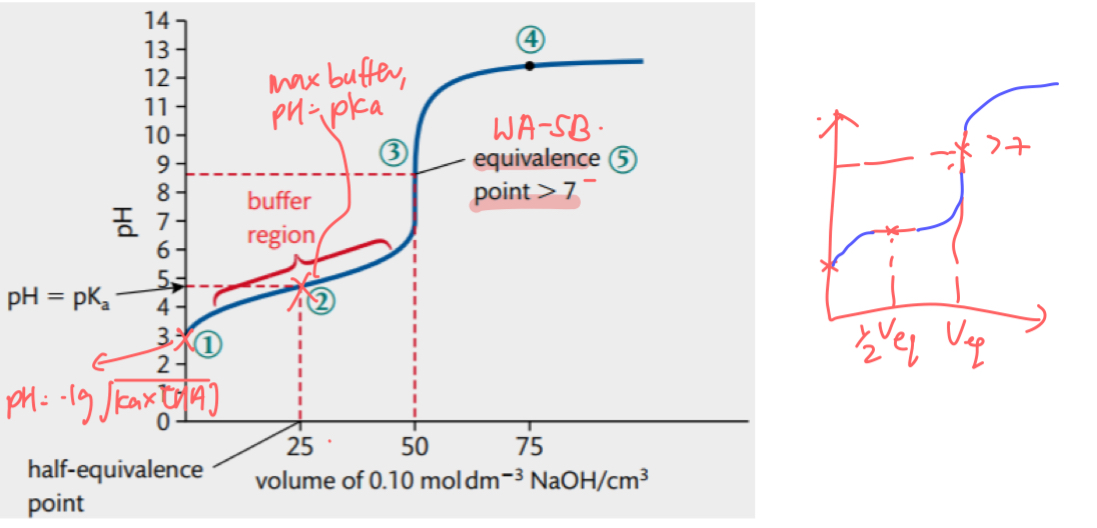
max buffer pH @ ½ Veq if before end point / 2 Veq if after end point → gradient horizontal
pH = pKa (if acidic buffer) / pOH = pKb
pH < 7: WA buffer (excess WB + limited SA)
pH > 7: WB buffer (excess WA + limited SB)
factors influencing max buffer
dilution: affects buffer capacity
reduces concentration of components → lowers buffer capacity
doesn’t change ratio → does not change pH of buffer solution
temperature: affects Ka/Kb → changes pH of buffer solution
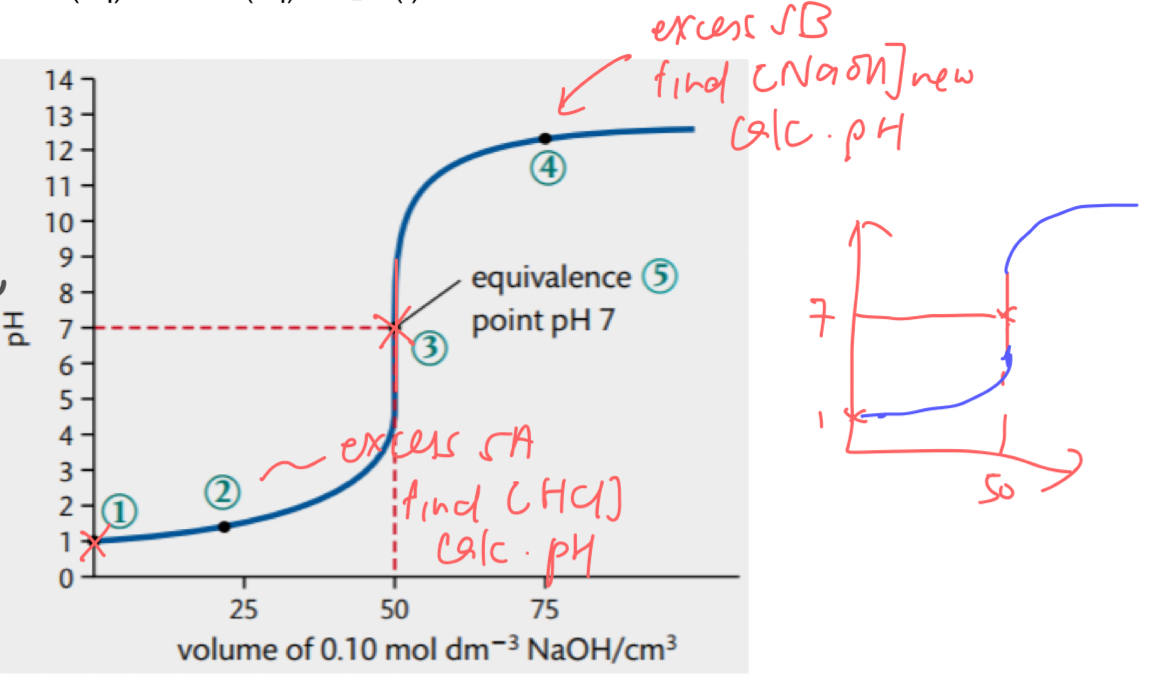
pH curve for SA SB
low initial pH due to SA
pH changes gradually until equivalence point
very sharp jump in pH at equivalence point → pH @ equivalence point = 7
after equivalence point, curve flattens out at high pH due to SB
pH curve for SA WB
low initial pH due to SA
pH changes until equivalence point reached
very sharp jump in pH at equivalence point → pH @ equivalence point < 7
after equivalence point pH increases gradually due to basic buffer (max buffer @ 2Veq)
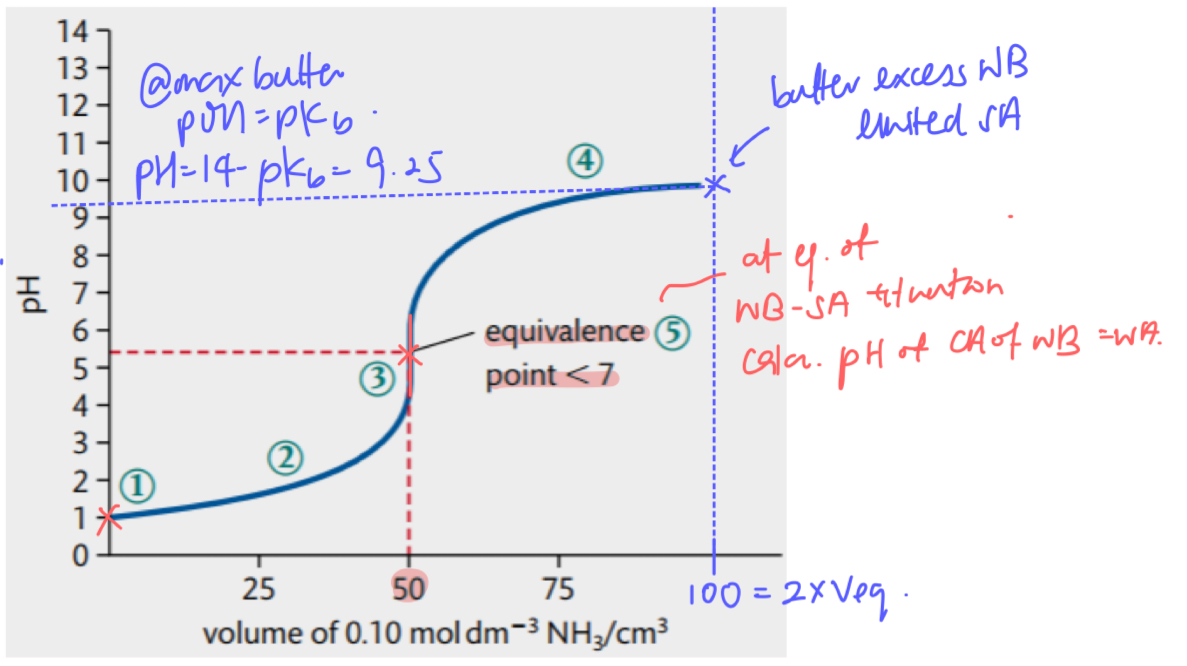
pH curve for WA SB
high initial pH due to WA
pH changes gradually until equivalence point due to acidic buffer
very sharp jump in pH at equivalence point → pH @ equivalence point > 7
after equivalence point the curve flattens out at high pH due to SB
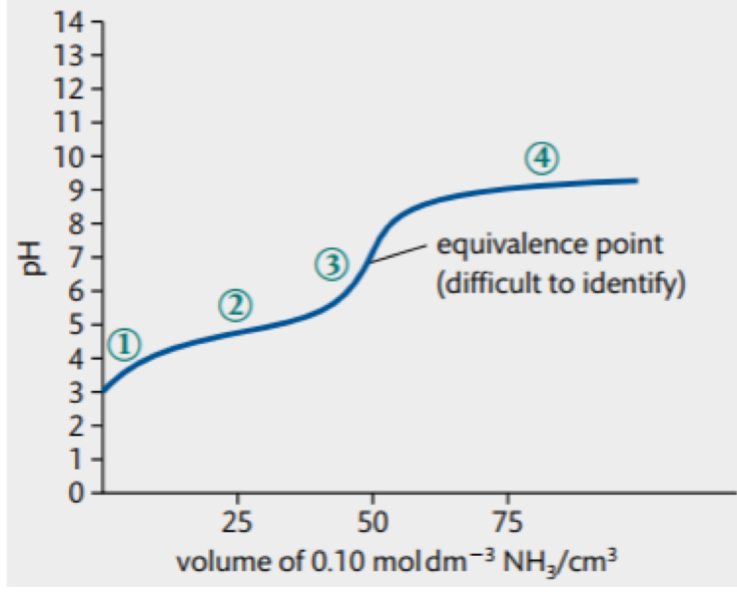
pH curve for WA WB
high initial pH due to WA
addition of base causes pH to rise steadily
change in pH at equivalence point is not sharp → unable determine
after equivalence point the curve flattens out at low pH due to WB
maximum buffer capacity
amount of base/acid that ca be absorbed without significant changes to the pH
indicators: definition, how it works, explain useful range
conjugate acid/base pair are different colours → undergo neutralisation, used to determine end point
usually weak organic acids with complex structures
HInd is one colour
Ind- (conjugate base) is another colour
HInd <=> H+ + Ind-
if [H+] increases, POE shifts left, forms more HInd
if [H+] decreases, POE shifts right, forms more Ind-
when orange, red = yellow → [HInd] = [Ind] → max buffer
useful pH range for colour change of indicator is pKa (indicator) +- 1
when [Ind-]/[HInd] = 1/10, will start to see colour change
to measure useful range: add alkali/acid 1.0cm3 at a time to solution containing indicator + buffer, and continuously measure pH using pH meter → pH at start of colour change + end of colour change is the range (need buffer so that pH does not change too rapidly when add 1.0cm3)
choosing indicator
working pH range should lie within the sharp pH change at eq point
determine whether reacting SA/SB/WB/WA → pH of equivalence point
if pH = 7, any indicator; if pH < 7, choose pKa <7; if pH > 7, choose pKa > 7
explaining choice: “active pH range of indicator coincides with the sharp change in pH at the equivalence point”
note: indicator range falls within actual range, not the other way around
how to determine acid requiring greatest volume of base for complete reaction?
determine total [H+], which depends on [acid] X basicity
ie 0.1M H3PO4 > 0.1M H2SO4 regardless of given pH (measure of H+ at a point)
methods to find vol needed for neutralisation
pH titration
thermometric titration
conductometric titration
indicator titration
State and explain whether CH3CH2NH3NO3 and CH3COONH4 will be acidic, basic or neutral respectively
acidic, neutral

what happens when Mg powder added to solution of NH4Cl?
dissociation of weak acid in water: NH4+ <=> NH3 + H+
acid-metal reaction (without spectator ion): Mg + 2H+ → Mg2+ + H2
overall: Mg + 2NH4+ → 2NH3 + H2
powder dissolves
effervescence produced
Is NH4Cl solution acidic, alkaline or neutral?
acidic. NH4+ is CA of WB NH3
assumptions in pH calculation
assumes all H+ ions in solution come from acid, not self-ionisation of water → Kw << concentration so [acid] @ equilibrium = [acid] @ initial
assumes it is purely weak acid in water, so [H3O+] = [A-] → can simplify and use [H+]²
explain difference in neutralisation reaction for strong vs weak acid
strong acid dissociates fully in water, higher initial [H+] → faster rate
note: stoichiometric ratio same so the amount of product in the end is the same
larger increase in temperature (recall energetics)
enthalpy change of neutralisation for SASB > WASB
some energy released is absorbed by WA to dissociate (endothermic rxn) and release more H+