chapter 6, the skeletal system
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
what tissue is the skeleton composed of
composed of:
cartilage
bone tissue
epithelium
nerve
blood forming tissue
adipose
dense connective tissue
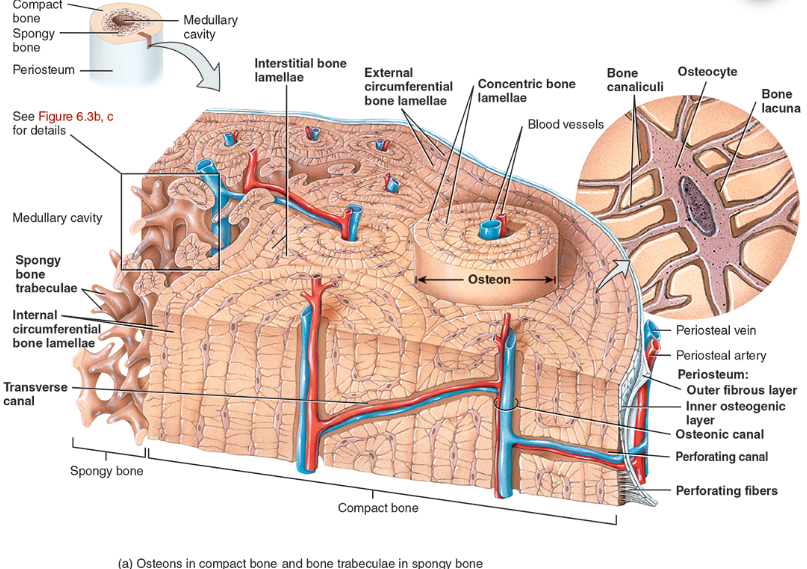
Perforating canal
A minute passageway by means of which blood vessels and nerves from the periosteum penetrate into compact bone.
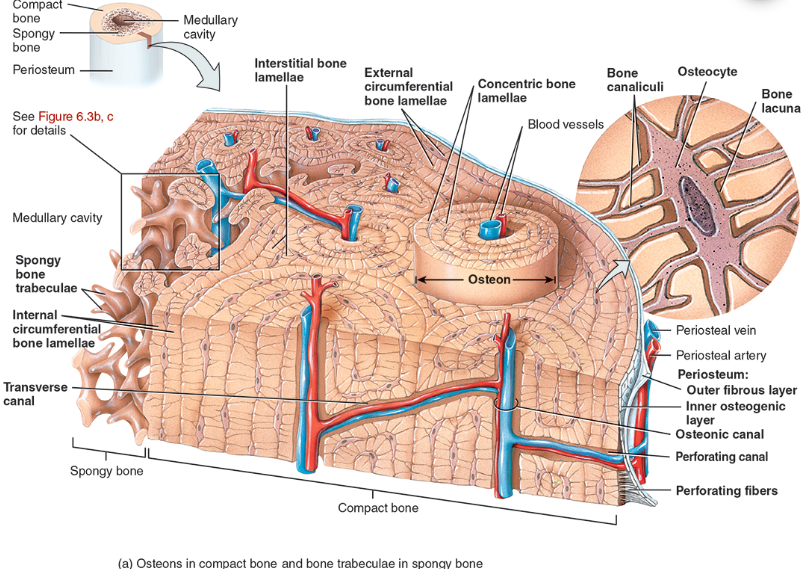
types of bone lamellae
interstitial bone lamellae
in the areas between neighboring osteons
are fragments of older osteons that have been partially destroyed during bone rebuilding or growth.
concentric bone lamellae
Resembling the growth rings of a tree
are circular plates of mineralized extracellular matrix of increasing diameter, surrounding a small network of blood vessels and nerves located in the osteonic canal
circumferential bone lamellae
Arranged around the entire outer and inner circumference of the diaphysis of a long bone
They develop during initial bone formation
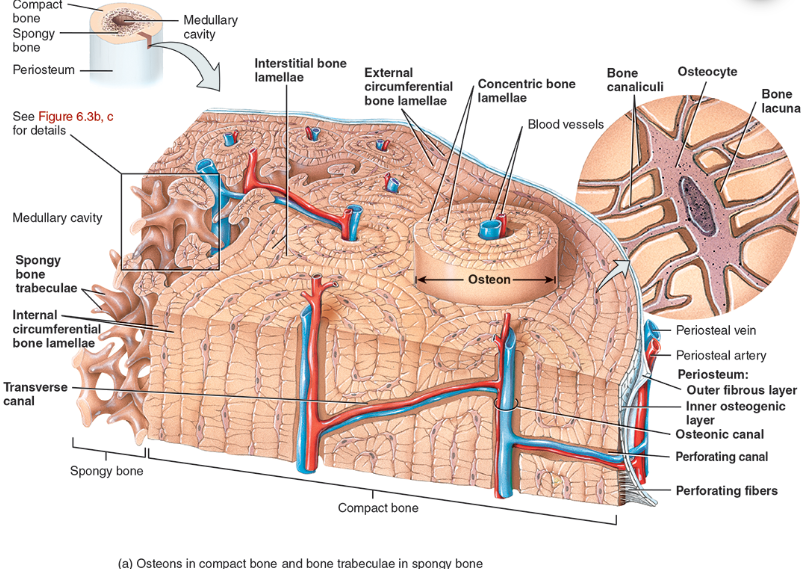
osteonic canal
A circular channel running longitudinally in the center of an osteon (haversian system) of mature compact bone, containing blood and lymphatic vessels and nerves.
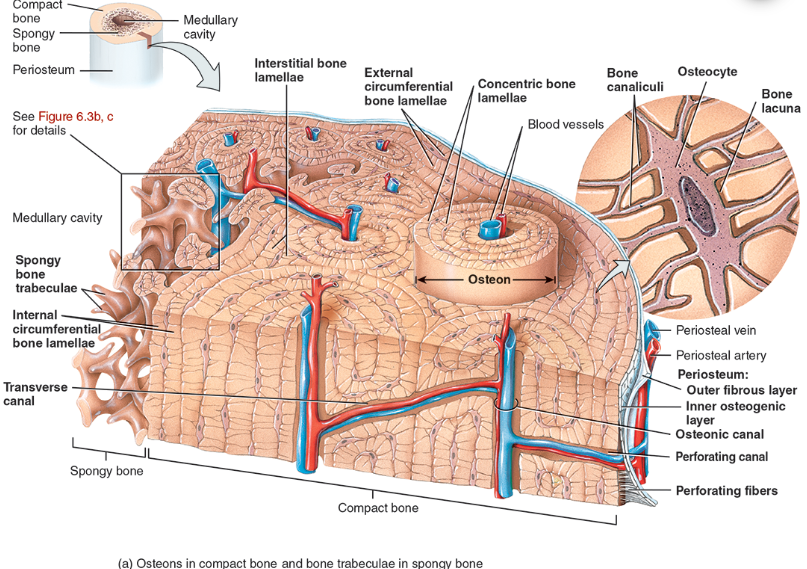
osteons (haversian systems)
The basic unit of structure in adult compact bone, consisting of a central canal with its concentrically arranged bone lamellae, bone lacunae, osteocytes, and bone canaliculi. Also called a haversian (ha‐VER‐shan) system.
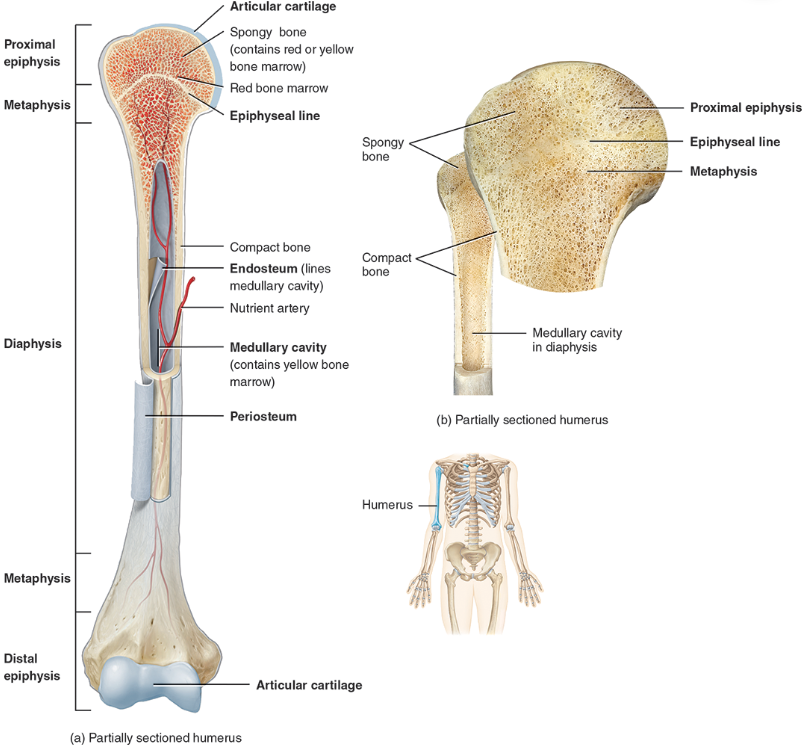
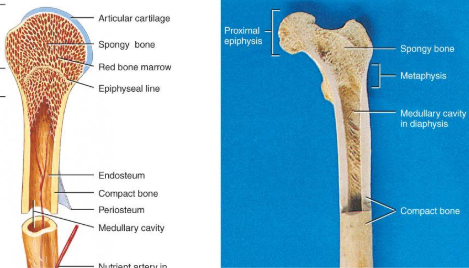
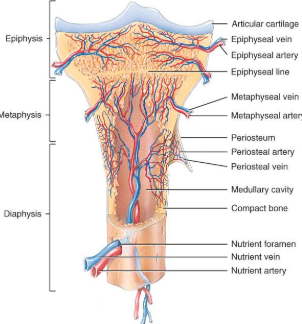
diaphysis
the bone’s—the long, cylindrical, main portion of the bone. It is also called the body or shaft
Epiphysis
The ends of a long bone, usually larger in diameter than the body (diaphysis).
Articular cartilage
Hyaline cartilage attached to articular bone surfaces.
avascular
reduces friction and absorbs shock
Periosteum
The membrane that covers bone and consists of connective tissue, osteoprogenitor cells, and osteoblasts; is essential for bone growth, repair, and nutrition.
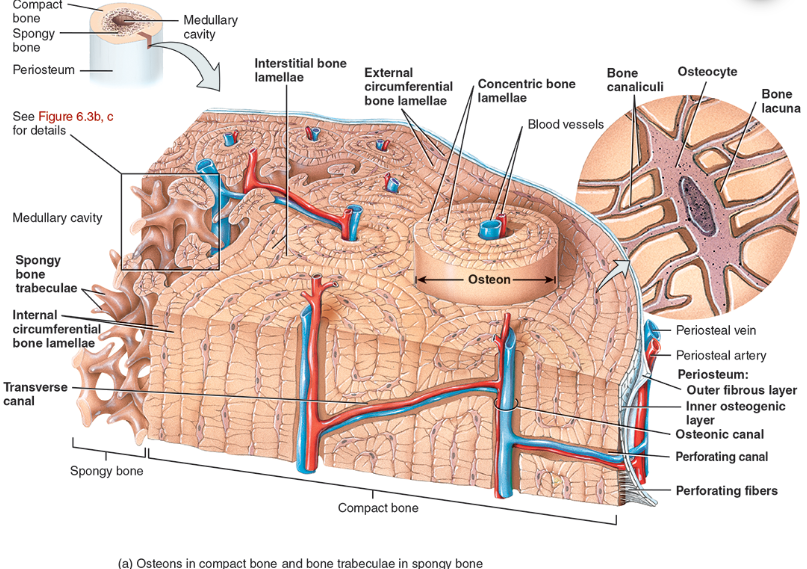
Perforating / sharpeys fibers
Thick bundles of collagen that extend from the periosteum into the bone extracellular matrix to attach the periosteum to the underlying bone.
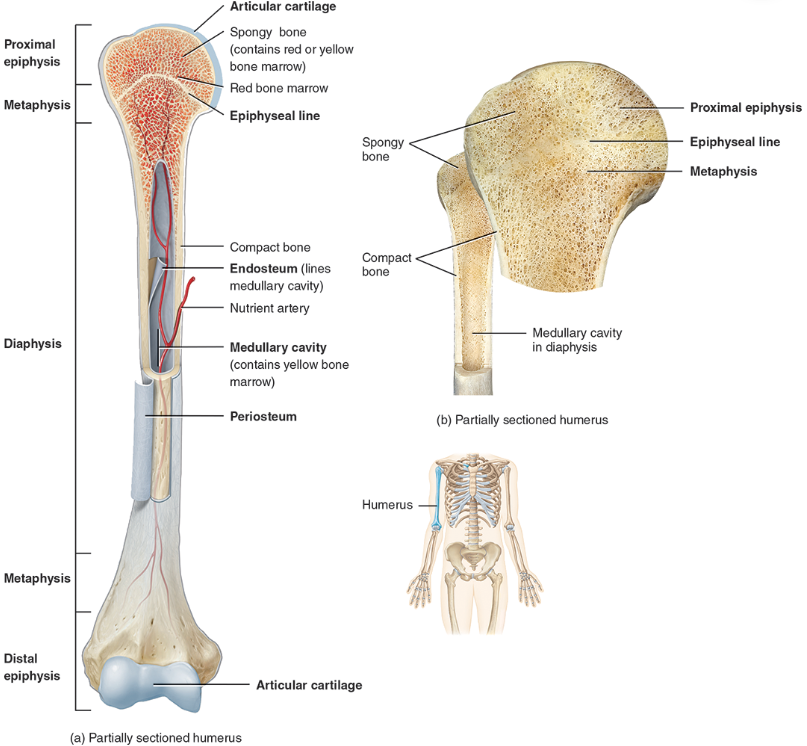
Medullary cavity
The space within the body of a bone that contains yellow bone marrow. Also called the marrow cavity.
what are functions of the bone
supporting and protecting soft tissues
Attachment site for muscles making movement possible
Storage of the minerals, calcium & phosphate -- mineral homeostasis
Blood cell production occurs in red bone marrow (hemopoiesis)
Energy storage in yellow bone marrow
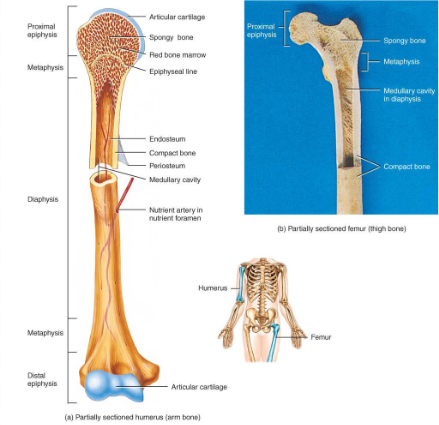
anatomy of a long bone
diaphysis = shaft
epiphysis = one end of a long bone
metaphyses are the areas between the epiphysis and diaphysis and include the epiphyseal plate in growing bones.
Articular cartilage over joint surfaces acts as friction reducer & shock absorber
Medullary cavity = marrow cavity
Endosteum = lining of marrow cavity
Periosteum = tough membrane covering bone but not the cartilage
fibrous layer = dense irregular CT
osteogenic layer = bone cells & blood vessels that nourish or help with repairs
histology of the bone
A type of connective tissue as seen by widely spaced cells separated by matrix
Matrix of 25% water, 25% collagen fibers & 50% crystalized mineral salts
4 types of cells in bone tissue
Bone (osseous) tissue consists of widely separated cells surrounded by large amounts of matrix.
The matrix of bone contains inorganic salts, primarily hydroxyapatite and some calcium carbonate, and collagen fibers.
These and a few other salts are deposited in a framework of collagen fibers, a process called calcification or mineralization.
The process of calcification occurs only in the presence of collagen fibers.
Mineral salts confer hardness on bone while collagen fibers give bone its great tensile strength.
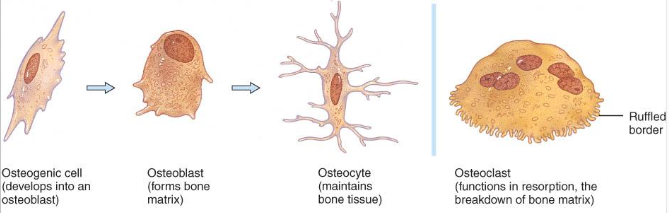
bone cells
Osteogenic cells undergo cell division and develop into osteoblasts.
Osteoblasts are bone-building cells.
Osteocytes are mature bone cells and the principal cells of bone tissue.
Osteoclasts are derived from monocytes and serve to break down bone tissue.
Osteoprogenitor cells
undifferentiated cells
can divide to replace themselves & can become osteoblasts
found in inner layer of periosteum and endosteum
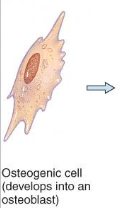
Osteoblasts
form matrix & collagen fibers but can’t divide
bone-building cells
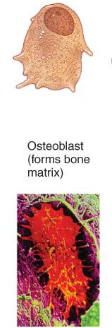
Osteocytes
mature cells that no longer secrete matrix
mature bone cells and the principal cells of bone tissue.
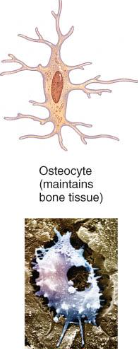
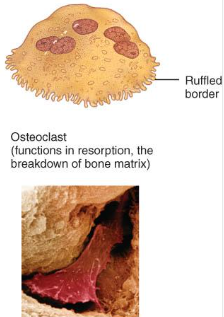
Osteoclasts
huge cells from fused monocytes (WBC)
function in bone resorption at surfaces such as endosteum
derived from monocytes and serve to break down bone tissue.
matrix of the bone
Inorganic mineral salts provide bone’s hardness
hydroxyapatite (calcium phosphate) & calcium carbonate
Organic collagen fibers provide bone’s flexibility
their tensile strength resists being stretched or torn
remove minerals with acid & rubbery structure results
Bone is not completely solid since it has small spaces for vessels and red bone marrow
spongy bone has many such spaces
compact bone has very few such spaces
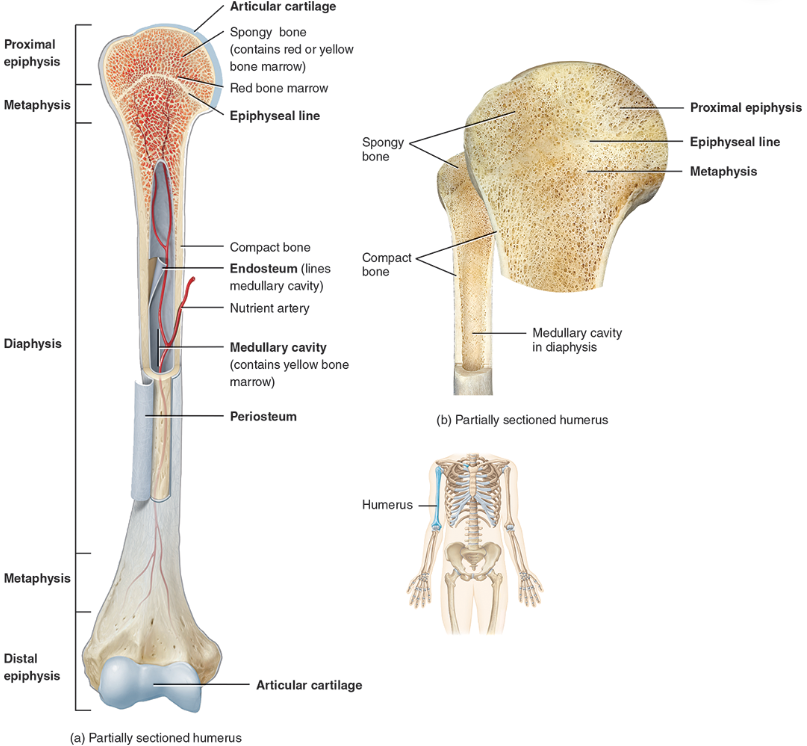
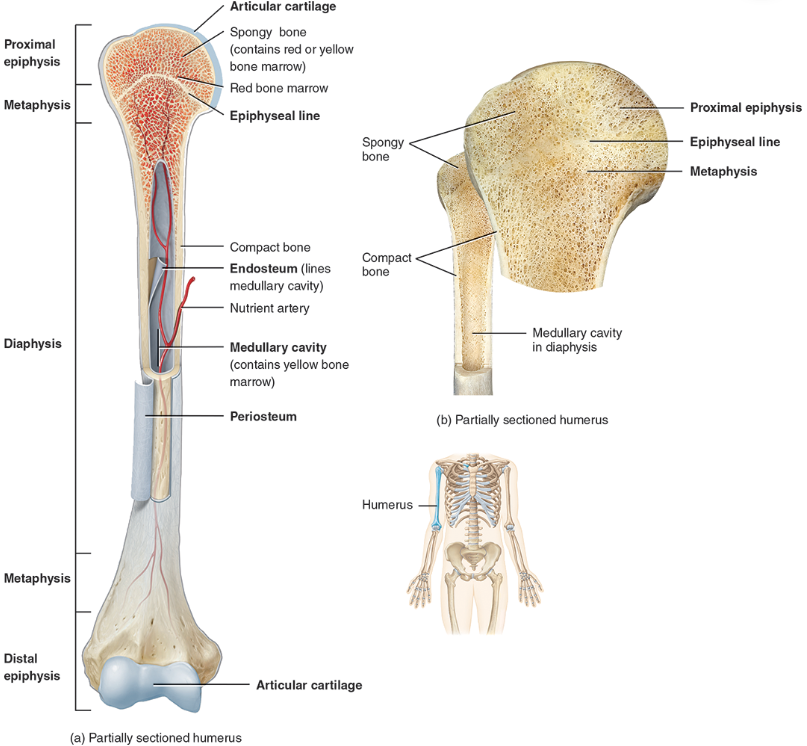
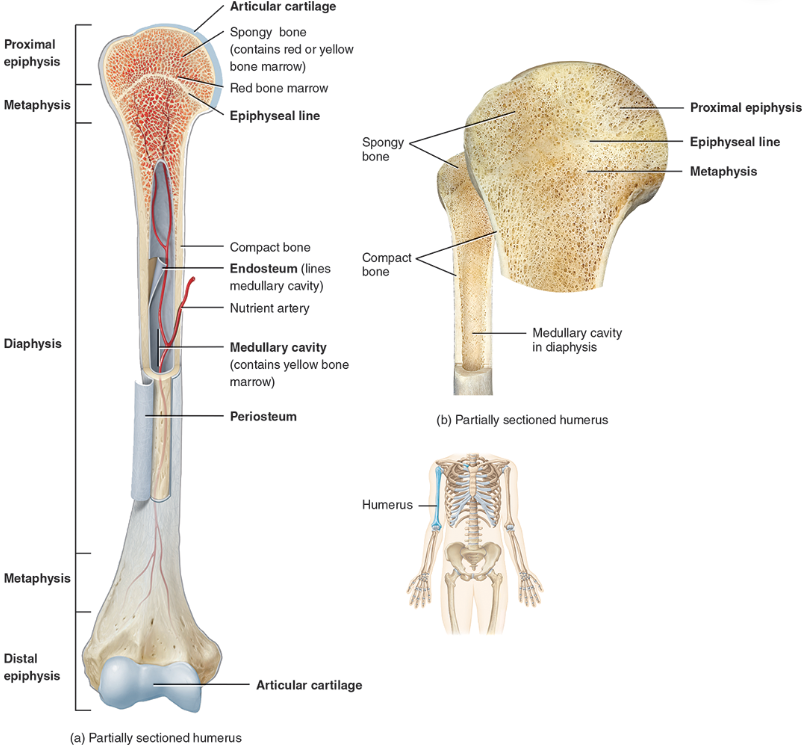
compact / dense bone
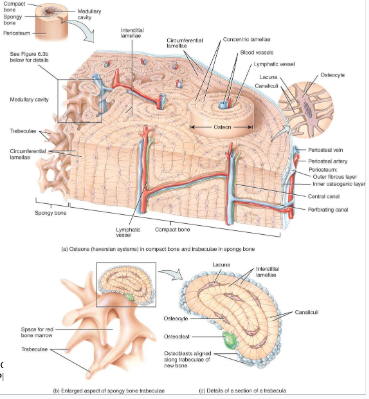
Compact bone is arranged in units called osteons or Haversian systems (Figure 6.3a).
Osteons contain blood vessels, lymphatic vessels, nerves, and osteocytes along with the calcified matrix.
Osteons are aligned in the same direction along lines of stress. These lines can slowly change as the stresses on the bone changes.
Looks like solid hard layer of bone
Makes up the shaft of long bones and the external layer of all bones
Resists stresses produced by weight and movement
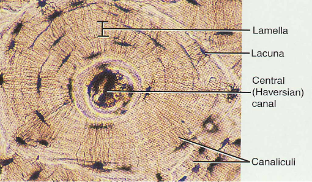
histology of compact bone
Osteon is concentric rings (lamellae) of calcified matrix surrounding a vertically oriented blood vessel
Osteocytes are found in spaces called lacunae
Osteocytes communicate through canaliculi filled with extracellular fluid that connect one cell to the next cell
Interstitial lamellae represent older osteons that have been partially removed during tissue remodeling
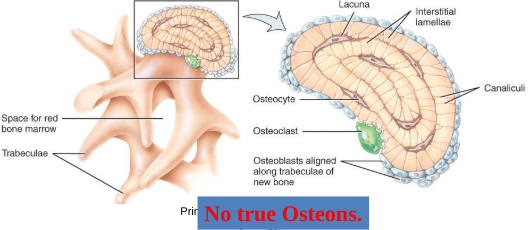
spongy bone
Spongy (cancellous) bone does not contain osteons. It consists of trabeculae surrounding many red marrow filled spaces (Figure 6.3b).
It forms most of the structure of short, flat, and irregular bones, and the epiphyses of long bones.
Spongy bone tissue is light and supports and protects the red bone marrow.
the trabeculae of spongy bone
Latticework of thin plates of bone called trabeculae oriented along lines of stress
Spaces in between these struts are filled with red marrow where blood cells develop
Found in ends of long bones and inside flat bones such as the hipbones, sternum, sides of skull, and ribs.
blood and nerve supply of bone
Periosteal arteries
supply periosteum
Nutrient arteries
enter through nutrient foramen
supplies compact bone of diaphysis & red marrow
Metaphyseal & epiphyseal aa.
supply red marrow & bone tissue of epiphyses
bone formation
All embryonic connective tissue begins as mesenchyme.
Bone formation is termed osteogenesis or ossification and begins when mesenchymal cells provide the template for subsequent ossification.
Two types of ossification occur.
Intramembranous ossification is the formation of bone directly from or within fibrous connective tissue membranes.
Endochondrial ossification is the formation of bone from hyaline cartilage models.
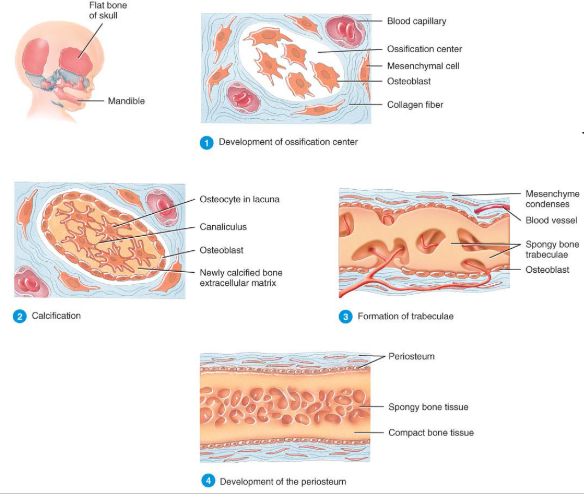
intramembranous
Intramembranous ossification forms the flat bones of the skull and the mandible (Figure 6.5).
An ossification center forms from mesenchymal cells as they convert to osteoblasts and lay down osteoid matrix.
The matrix surrounds the cell and then calcifies as the osteoblast becomes an osteocyte.
The calcifying matrix centers join to form bridges of trabeculae that constitute spongy bone with red marrow between.
On the periphery the mesenchyme condenses and develops into the periosteum.
Mesenchymal cells become osteoprogenitor cells then osteoblasts.
Osteoblasts surround themselves with matrix to become osteocytes.
endochondrial ossification
Endochondrial ossification involves replacement of cartilage by bone and forms most of the bones of the body (Figure 6.6).
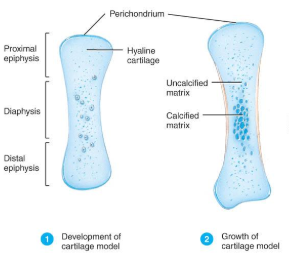
The first step in endochondrial ossification is the development of the cartilage model.
Development of Cartilage model (endochondral bone formation)
Mesenchymal cells form a cartilage model of the bone during development
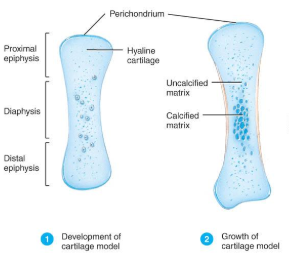
Growth of Cartilage model (Endochondral Bone Formation
in length by chondrocyte cell division and matrix formation ( interstitial growth)
in width by formation of new matrix on the periphery by new chondroblasts from the perichondrium (appositional growth)
cells in midregion burst and change pH triggering calcification and chondrocyte death
Development of Primary Ossification Center (Endochondral Bone Formation)
perichondrium lays down periosteal bone collar
nutrient artery penetrates center of cartilage model
periosteal bud brings osteoblasts and osteoclasts to center of cartilage model
osteoblasts deposit bone matrix over calcified cartilage forming spongy bone trabeculae
osteoclasts form medullary cavity
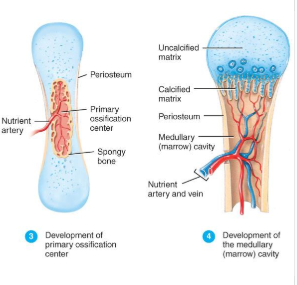
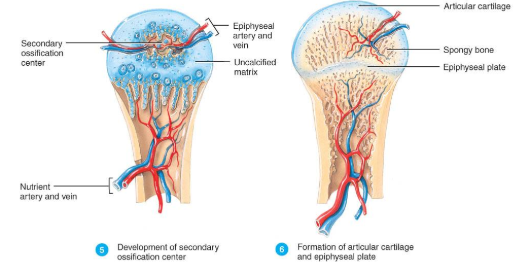
Development of Secondary Ossification Center and Formation of Articular Cartilage (Endochondral Bone Formation)
Development of Secondary Ossification Center
blood vessels enter the epiphyses around time of birth
spongy bone is formed but no medullary cavity
Formation of Articular Cartilage
cartilage on ends of bone remains as articular cartilage.
scanning of the bone
Radioactive tracer is given intravenously
Amount of uptake is related to amount of blood flow to the bone
“Hot spots” are areas of increased metabolic activity that may indicate cancer, abnormal healing or growth
“Cold spots” indicate decreased metabolism of decalcified bone, fracture or bone infection
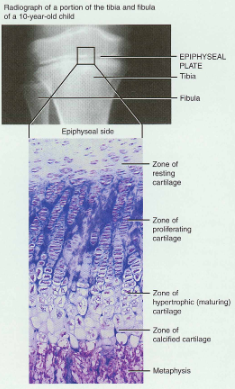
bone growth in length
To understand how a bone grows in length, one needs to know details of the epiphyseal or growth plate (Figure 6.7).
The epiphyseal plate consists of four zones
When the epiphyseal plate closes, is replaced by bone, the epiphyseal line appears and indicates the bone has completed its growth in length.
Epiphyseal plate or cartilage growth plate
cartilage cells are produced by mitosis on epiphyseal side of plate
cartilage cells are destroyed and replaced by bone on diaphyseal side of plate
Between ages 18 to 25, epiphyseal plates close.
cartilage cells stop dividing and bone replaces the cartilage (epiphyseal line)
Growth in length stops at age 25
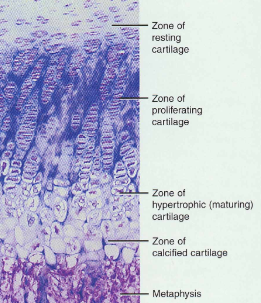
Zones of Growth in Epiphyseal Plate
Zone of resting cartilage
anchors growth plate to bone
Zone of proliferating cartilage
rapid cell division (stacked coins)
Zone of hypertrophic cartilage
cells enlarged & remain in columns
Zone of calcified cartilage
thin zone, cells mostly dead since matrix calcified
osteoclasts removing matrix
osteoblasts & capillaries move in to create bone over calcified cartilage
bone growth in thickness
Bone can grow in thickness or diameter only by appositional growth (Figure 6.8).
The steps in thes process are:
Periosteal cells differentiate into osteoblasts which secrete collagen fibers and organic molecules to form the matrix.
Ridges fuse and the periosteum becomes the endosteum.
New concentric lamellae are formed.
Osetoblasts under the peritsteum form new circumferential lamellae.
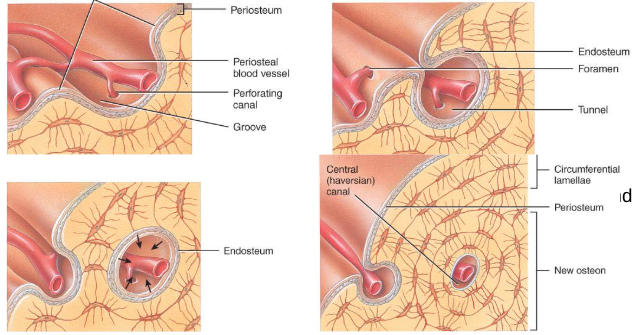
bone growth in width
Only by appositional growth at the bone’s surface
Periosteal cells differentiate into osteoblasts and form bony ridges and then a tunnel around periosteal blood vessel.
Concentric lamellae fill in the tunnel to form an osteon.
factors affecting bone growth
Nutrition
adequate levels of minerals and vitamins
calcium and phosphorus for bone growth
vitamin C for collagen formation
vitamins K and B12 for protein synthesis
Sufficient levels of specific hormones
during childhood need insulinlike growth factor
promotes cell division at epiphyseal plate
need hGH (growth), thyroid (T3 &T4) and insulin
sex steroids at puberty
At puberty the sex hormones, estrogen and testosterone, stimulate sudden growth and modifications of the skeleton to create the male and female forms.
hormonal abnormalities with bones
Oversecretion of hGH during childhood produces giantism
Undersecretion of hGH or thyroid hormone during childhood produces short stature
Both men or women that lack estrogen receptors on cells grow taller than normal
estrogen is responsible for closure of growth plate
bone remodeling
Remodeling is the ongoing replacement of old bone tissue by new bone tissue.
Old bone is constantly destroyed by osteoclasts, whereas new bone is constructed by osteoblasts.
In orthodontics teeth are moved by brraces. This places stress on bone in the sockets causing osteoclasts and osteablasts to remodel the sockets so that the teeth can be properly aligned (Figure 6.2)
Several hormones and calcitrol control bone growth and bone remodeling (Figure 6.11)
Ongoing since osteoclasts carve out small tunnels and osteoblasts rebuild osteons.
osteoclasts form leak-proof seal around cell edges
secrete enzymes and acids beneath themselves
release calcium and phosphorus into interstitial fluid
osteoblasts take over bone rebuilding
Continual redistribution of bone matrix along lines of mechanical stress
distal femur is fully remodeled every 4 months
fracture and repair of bone
A fracture is any break in a bone.
Fracture repair (Figure 6.10)involves formation of a clot called a fracture hematoma, organization of the fracture hematoma into granulation tissue called a procallus (subsequently transformed into a fibrocartilaginous [soft] callus), conversion of the fibrocartilaginous callus into the spongy bone of a bony (hard) callus, and, finally, remodeling of the callus to nearly original form.
Healing is faster in bone than in cartilage due to lack of blood vessels in cartilage
Healing of bone is still slow process due to vessel damage
Clinical treatment
closed reduction = restore pieces to normal position by manipulation
open reduction = realignment during surgery
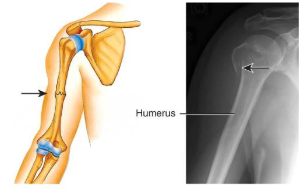
greenstick fracture
partial fracture
impacted fracture
one side of fracture driven into the interior of other side
closed fracture
no break in skin
open fracture
skin broken
comminuted fracture
broken ends of bones are fragmented
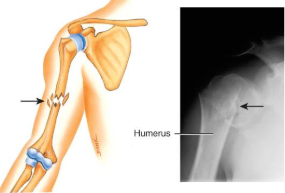
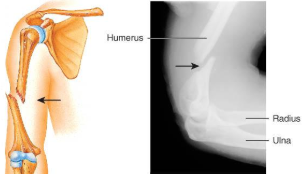
potts fracture
distal fibular fracture
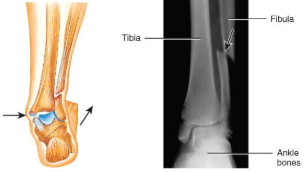
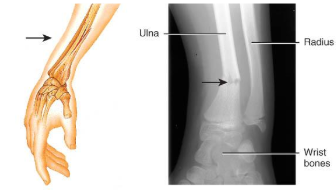
colles’s fracture
distal radial fracture
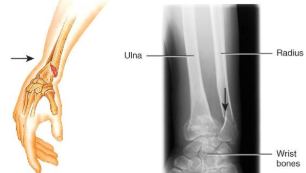
stress fracture
microscopic fissures from repeated strenuous activities
repair of a fracture
Formation of fracture hematoma
damaged blood vessels produce clot in 6-8 hours, bone cells die
inflammation brings in phagocytic cells for clean-up duty
new capillaries grow into damaged area
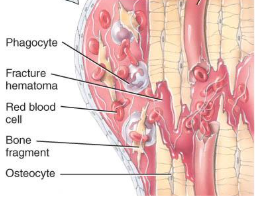
repair of a fracture
Formation of fibrocartilagenous callus formation
fibroblasts invade the procallus & lay down collagen fibers
chondroblasts produce fibrocartilage to span the broken ends of the bone
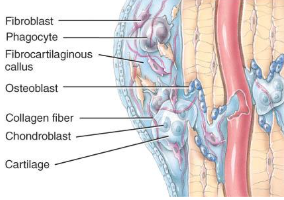
repair of a fracture
Formation of bony callus
osteoblasts secrete spongy bone that joins 2 broken ends of bone
lasts 3-4 months
repair of a fracture
Bone remodeling
compact bone replaces the spongy in the bony callus
surface is remodeled back to normal shape
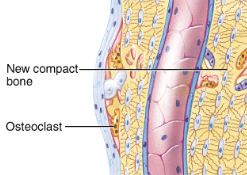
calcium homeostasis and bone tissue
Skeleton is a reservoir of Calcium & Phosphate
Calcium ions involved with many body systems
nerve & muscle cell function
blood clotting
enzyme function in many biochemical reactions
Small changes in blood levels of Ca+2 can be deadly (plasma level maintained 9-11mg/100mL)
cardiac arrest if too high
respiratory arrest if too low
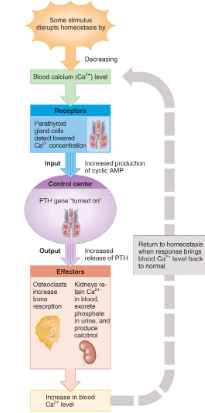
hormonal influences
Parathyroid hormone (PTH) is secreted if Ca+2 levels falls
PTH gene is turned on & more PTH is secreted from gland
osteoclast activity increased, kidney retains Ca+2 and produces calcitriol
Calcitonin hormone is secreted from parafollicular cells in thyroid if Ca+2 blood levels get too high
inhibits osteoclast activity
increases bone formation by osteoblasts
exercise and bone tissue
Within limits, bone has the ability to alter its strength in response to mechanical stress by increasing deposition of mineral salts and production of collagen fibers.
Removal of mechanical stress leads to weakening of bone through demineralization (loss of bone minerals) and collagen reduction.
reduced activity while in a cast
astronauts in weightless environment
bedridden person
Weight-bearing activities, such as walking or moderate weightlifting, help build and retain bone mass.
the development of bone tissue
Both types of bone formation begin with mesenchymal cells
Mesenchymal cells transform into chondroblasts which form cartilage
OR
Mesenchymal cells become osteoblasts which form bone
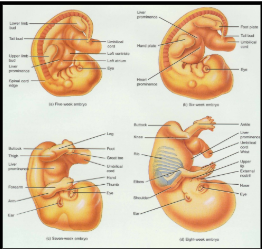
developmental anatomy
5th Week =limb bud appears as mesoderm covered with ectoderm
6th Week = constriction produces hand or foot plate and skeleton now totally cartilaginous
7th Week = endochondral ossification begins
8th Week = upper & lower limbs appropriately named
aging and bone tissue
Of two principal effects of aging on bone, the first is the loss of calcium and other minerals from bone matrix (demineralization), which may result in osteoporosis.
very rapid in women 40-45 as estrogens levels decrease
in males, begins after age 60
The second principal effect of aging on the skeletal system is a decreased rate of protein synthesis
decrease in collagen production which gives bone its tensile strength
decrease in growth hormone
bone becomes brittle & susceptible to fracture
osteoporosis
Decreased bone mass resulting in porous bones
Those at risk
white, thin menopausal, smoking, drinking female with family history
athletes who are not menstruating due to decreased body fat & decreased estrogen levels
people allergic to milk or with eating disorders whose intake of calcium is too low
Prevention or decrease in severity
adequate diet, weight-bearing exercise, & estrogen replacement therapy (for menopausal women)
behavior when young may be most important factor
Disorders of Bone Ossification
Rickets
calcium salts are not deposited properly
bones of growing children are soft
bowed legs, skull, rib cage, and pelvic deformities result
Osteomalacia
new adult bone produced during remodeling fails to ossify
hip fractures are common