MCAT: General Chemistry Chapter 1 (KAPLAN)
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
isotopes
different number of neutrons in atoms, same atomic number but have different mass number -- they exhibit similar chemical properties and are normally labeled by their mass number (carbon 12) except in hydrogens (protium, deuterium, and tritium)
mass number
sum of protons and nucleus in nucleus -- changes with each varying isotope
valence electrons
farthest electrons from the nucleus and because they are so far, they feel the least amount of electrostatic pull from the nucleus and therefore can bond with others (interact with their surrounding environment) -- determine reactivity of an atom
most easily removed
"active" electrons
dominate the chemical behavior of an atom
Electron energy levels
electrons closer to the cell nucleus have lower energy levels, and farther away have higher energy levels
which subatomic particle is most important for determining: charge?
electron
which subatomic particle is most important for determining: atomic number?
proton
which subatomic particle is most important for determining: isotope?
neutron
how to determine number of protons
atomic number
atomic weight
average mass of naturally occurring isotopes
mass of one proton
one amu = 1/12 of the carbon-12 atom
(difference between proton and neutron mass is very small, the difference is equal to an electron)
half-life correspond with ____ therefore helps determine ____
stability, the relative proportions of these different isotopes
the relationship between atomic weight, isotopes, and moles
the atomic weight of carbon is 12 amu, which means the average carbon weighs 12 amu (meaning carbon 12 isotope is extremely more abundant than 13 or 14) and one mole of carbon atoms is equal to 12 grams
atomic mass
nearly equal to mass number (slightly less than the number of protons and neutrons in a nucleus)
Ernest Rutherford
experimental evidence that an atom has a dense positively charged nucleus that one accounts for a small portion of the atom's volume (VOLUME)
Max Planck
developed first quantum theory - energy emitted as electromagnetic radiation for matter comes in discrete bundles called quanta (the energy of a quantum is given by the Planck Relation)
Plank Relation
E=h*f
Planck's constant
h, frequency of the radiation (in Planck's relation)
Neils Bohr
electron traveled in a circular orbit around the central proton nucleus, this centripetal force acting on the electron is from the electrostatic force between positive protons and negative electrons (used the work of Rutherford and Planck)
Bohr's use of Planck's constant
placed restrictions on the angular momentum that drove the pathways of electrons (previously pathways were defined by classic physics)
L=(n*h)/2pi
because n is the only variable, the angular momentum can only change in discrete amounts respective to the quantum number
what are the similarities between quantized angular momentum and Planck's concept of quantized energy
There is only discrete energy levels possible, energy isn't infinite
Bohr related the permitted angular momentum values to the energy of the electron to obtain:
the energy of the electron changes in discrete amounts with respect to the quantum number (im sure the actual equation is not important to memorize E=-Rn/n^2)
what is the energy of the electron equation saying? (E=-Rn/n^2)
as the energy of an electron increases, aka becomes less negative, the farther out from the nucleus it will be located (larger n)
important point: while magnitude of the fraction is getting smaller, the actual value it represents is getting larger (becoming less negative)
Rn
Rydberg unit of energy
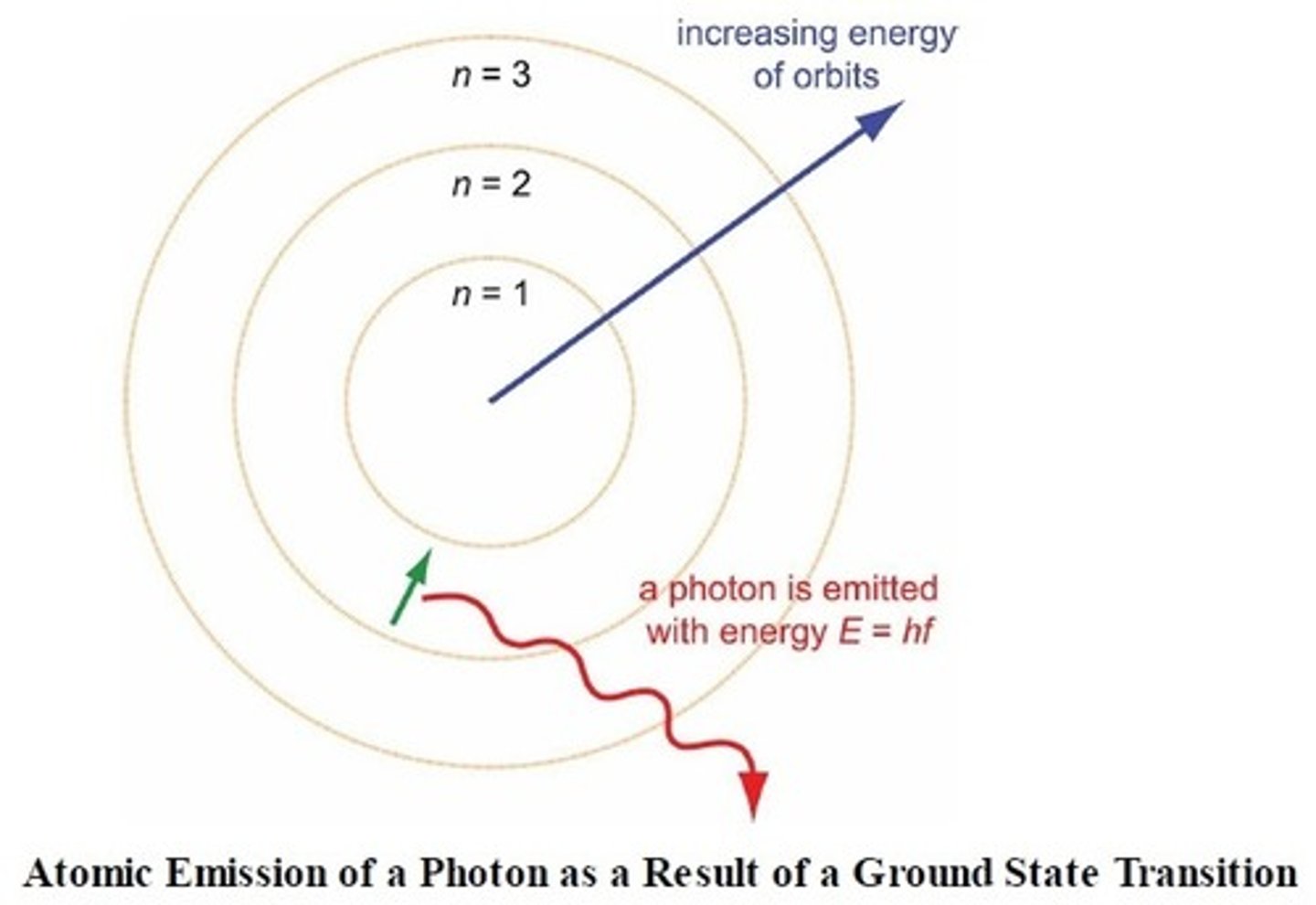
ground state
n=1 (lowest and smallest energy radius)
electron orbit
bohr said that electrons revolved in a defined pathway at a discrete energy value, and that with the transfer of energy (gaining) they could "jump" to higher energy orbits or "fall" with the loss of energy (he likened the electrons orbiting the nucleus the way the planets do with the sun)
excited state
when at least one electron in an atom is at a higher energy level than normal
how was Bohr wrong? why is he important/useful?
important for conceptualization of atomic behavior, but he was wrong because electrons are not restricted to specific pathways, they tend to be localized in certain regions of space. It is still useful for explaining atomic emission and absorption spectra of atoms
all systems tend toward ________ _________
minimal energy (lowest energy = more stability)
atomic emission spectra
E=(h*c)/lamda
h
planck's constant
c
speed of light
lambda
wavelength
when electrons return close to their ground states, they will
emit a photon with a wavelength characteristic of the specific energy transition it undergoes
atomic emission spectrum
each element has unique emissions from when the electrons change energy levels (because they all have distinct energy levels) so that line spectrum can be used as a fingerprint for that element
Lyman series
transition from energy level n=1 to energy level n=2 or higher -- larger energy transitions so shorter photon wavelengths (i dont get that ask doctor nataro page 13)
Balmer series
transition from energy level n=2 to energy level n=3 or higher
Paschen series
transition from energy level n=3 to energy level n=4 or higher
combining Bohr's and Planck's calculations we derive a complex-appearing equation that says
the energy of the emitted photon corresponds to the difference in energy between the higher-energy initial state and lower-energy final state
positive E correlates to _____ and negative E from the equation correlates to _______
emission, absorption
atomic emission versus absorption spectrum
for an element, the wavelengths of the emission and absorption (to "fall" or "jump" and energy level) are the same
each element has a characteristic set of energy levels
atomic absorption spectrum
useful for identifying elements in the gas phase, energy at a specific wavelength absorbed causes an "energy jump"
energy is absorbed for an electron to "jump" levels through ____ and emitted in the same form when they "fall" levels
light
Bohr's model failure
not taking into account the repulsion between multiple electrons surrounding the nucleus (this is why his model failed when explaining the structure and behavior of atoms with more than one electron)
most important difference between Bohr's model and modern quantum mechanical model
Bohr postulated that electrons follow a clearly defined circular pathway, or orbit at a fixed distance from the nucleus
modern: electrons move rapidly and are localized within regions of space around the nucleus called orbitals
the best we can do now (bohr's time thought we could identify the location or pathway of an electron) is more modest:
describe the probability of finding an electron within a given region of space surrounding the nucleus
Heisenberg uncertainty principle
it is impossible to simultaneously determine, with perfect accuracy, the momentum and position of an electron
to determine momentum the electron has to be moving
to determine position the electron must stop (confused by this ask nataro page 16)
any electron in an atom can be completely described by 4 quantum numbers:
n, l ,ml , ms
Pauli exclusion principle
no two electrons in a given atom can possess the same set of four quantum numbers
energy state
position and energy of an electron described by its quantum numbers
the relationship between n, l, ml, ms
the value of n limits the value of l, which in turn limits the value of ml, ms is either +/- 1/2`
principal quantum number (n)
the larger the integer value of n, the higher the energy level and radius of the electron's shell (within each shell there is a capacity to hold a certain number of electrons)
maximum number of electrons within a shell
2n^2
difference in energy levels
the distance between n=1 and n=2 is the largest (when only moving one level) n=3 and n=4 is smaller, this makes sense when you think of 1/(n-i)^2 - 1/(n-f)^2
azimuthal quantum number (l)
shape and number of sub shells within a principle energy level (shell)
important implications for chemical bonding and bond angles
how does n limit l
the values for l are any integers between 0 and n-1 (so there is only one subshell in n=1 --> value of n tells you how many subshells there are)
spectroscopic notation
s (l=0) p (l=1) d (l=2) f (l=3)
if n=4 and l=2 then it is 4d
if n=3 and l=0 then it is 3s
maximum number of electrons within a seubshell
4l + 2 (ask dr. nataro page 18)
magnetic quantum number (ml)
specifies the particular orbital within a sub shell where an electron is most likely to be found at a given moment in time
the possible values are between -1 and +1 (including 0)
possible ml equation
2l+1
for any n, this produces ___ orbitals and a maximum of ____ electrons (two per orbital)
n^2, 2n^2
probability density
likelihood that an electron will be found in a particular region of space
as you go left to right (--->) on the periodic table what happens? (electrons)
an electron is added to the orbital)
spin quantum number (ms)
electron has two spin orientations designated +1/2 and -1/2
paired electrons
whenever two electrons are in the same orbital, they must have opposite spins
parallel spins
electrons in different orbitals with the same ms values
electron configuration (ex: 2p^4)
the pattern by which subshells are filled, as well as the number of electrons within each principal energy level and subshell
aufbau principle (building-up principle)
electrons fill from lower to higher-energy subshells and each subshell will completely fill before electrons begin to enter the next one
n + l rule (important for test day)
the lower the sum of the values of the first and second quantum numbers, the lower the energy of the subshell
if two subshells possess the same n + l value (important for test day)
the subshell with the lower n value has a lower energy and will fill with electrons first
electron configuration of an ion
anion / F-
cation / Fe3+
anion: electrons fill the same way that they normally would
[He]2s^2 2p^6
cation: start with the neutral atom and remove electrons from the subshells with the highest n value first, then electrons are removed from the subshell with the highest l value among these
[Ar]3d^5
In subshells that contain more than one orbitals, the orbital fills in accordance to this rule
Hund's Rule
Hund's Rule (bus rule)
within a given subshell, orbitals are filled such that there are a maximum number of half-filled orbitals with parallel spins (they fill so each has their own orbital before doubling up, like a school bus)
why does hund's rule happen that way?
electron repulsion, electrons in the same orbital are closer to each other so they repel each other more than electrons in separate orbitals
a consequence of Hund's rule is
half-filled and fully filled orbitals have lower energies (higher stability) than other states
the consequence of Hund's rule creates two notable exceptions to electron configurtoin that are often tested on the MCAT:
chromium (and other elements of that group) and copper (and other elements in its group)
3d^4 becomes 3d^5
3d^9 becomes 3d^10
paramagnetic materials
composed of atoms with unpaired electrons that orient their spins in alignment with a magnetic field, so the material is weakly attracted to the magnetic field
PARAmagnetic = magnetic field that causes PARAllel spins in unpaired electrons and therefore cause an attraction
diamagnetic materials
atoms that only have paired electrons will be slightly repelled by a magnetic field
valence electrons for Group IA and IIA
the highest s subshell electrons
valence electrons for Group IIIA through VIIIA (13-18)
the highest s and p subshell electrons
valence electrons for transition elements
in the highest s and d subshells (even though they have different principal quantum numbers)
valence electrons for lanthanide and actinide series
in the highest s and f subshells (even though they have different principal quantum numbers)
how do elements in period three (starting with sodium) and below violet the octet rule? (will be discussed more in chapter 3)
they may accept electrons into their d subshells, which allows them to hold more than eight electrons in their valence shell