Unit 4: Acids and Bases (Part 1)
1/24
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Arrhenius Theory
An acid is any compound that produces hydrogen ions, H+(aq), in water.
→ Ex: HCl(g) → H+(aq) + Cl-(aq)A base is any compound that produces hydroxide ions, OH-(aq), in water.
→ Ex: NaOH(s) → Na+(aq) + OH-(aq)Note: Organic acids have formulas that end in “COOH”; do not confuse them with bases.
Neutralization reaction
A neutralization is simply a special case of a double replacement reaction in which water is always one of the products.
Acid + Base → Salt + Water
Properties of ACIDS
Taste SOUR
Conduct an electric current
Turn litmus paper RED
Produce hydrogen gas (H2) when reacted with certain metals, such as magnesium
Neutralized by bases
When an acid such as HCl is dissolved in water, it produces a hydrogen ion or proton, H+(aq).
Protons do not exist on their own in water, but rather readily attach themselves to water molecules (H2O) to produce hydronium ions or hydrated protons, H3O+(aq).
HCl(g) → H+(aq) + Cl-(aq)
HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
Properties of BASES
Taste like “SOAP”
Conduct an electric current
Turn litmus paper BLUE
Feel slippery
Neutralized by acids
Bronsted-Lowry Theory
ACID is any substance that can DONATE a proton (H+) to another substance.
→ Proton donorBASE is any substance that can ACCEPT a proton (H+) from another substance.
→ Proton acceptor
HCN(g) + H2O(l) ⇌ H3O+(aq) + CN-(aq)
Forward reaction:
HCN is an acid: it loses “H” and a “+1” charge to become CN-.
H2O is a base: it gains “H” and a “+1” charge to become H3O+.
Reverse reaction: H3O+ is the acid and CN- is the base.
Conjugate Acid-Base Pairs
A pair of chemicals that differ by only ONE PROTON.
CONJUGATE ACID has an extra proton.
CONJUGATE BASE lacks a proton.
HCN(g) + H2O(l) ⇌ H3O+(aq) + CN-(aq)
HCN and CN- are the conjugate acid-base pair
HCN is the acid, and CN- is the conjugate base.
H2O is the base, and H3O+ is the conjugate acid.
Amphiprotic
Substances that can act as either an acid or a base, depending on the kind of substances they react with.
HCN(g) + H2O(l) ⇌ H3O+(aq) + CN-(aq)
H2O gains a proton to become H3O+, so it is acting as a BASE.
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
H2O loses a proton to become OH-, so it is acting as an ACID.
→ Water can be considered AMPHIOROTIC.
Recognizing amphiprotic substances
A substance is amphiprotic if it:
possesses a NEGATIVE CHARGE
still has an easily removable HYDROGEN*
Strengths of acids and bases
STRONG acids and bases ionize completely, 100% IONIZED.
WEAK acids and bases do not ionize completely, LESS THAN 100% IONIZED.
→ Weak acids are better represented by an equilibrium system where the conjugate acid and base pairs both exist.The terms strong or weak refer to the degree of ionization of the acid or base, not the molar concentration.
→ Ex: 0.0010 M HCl is a STRONG acid, while 6.0 M HF is a WEAK acid.
Strong ACIDS
HBr, HCl, HI, HNO3, HClO4, H2SO4
All six reactions have ONE-WAY reaction arrows, pointing to the right.
Aqueous molecules of the acid do not exist, and the conjugate bases cannot accept a proton from water.
Ex: HCl(aq) + H2O(l) → H3O+(aq) + Cl-(aq)
Strong BASES
O2 and NH2
These two reactions have ONE-WAY reaction arrows, pointing to the left.
Aqueous molecules of the base do not exist, and the conjugate acids cannot donate a proton to water.
NH3(aq) + OH-(aq) ← NH2-(aq) + H2O(l)
Hydroxides are also strong bases: NaOH, LiOH, KOH, Ca(OH)2, Ba(OH)2, Sr(OH)2
Weak ACIDS
Weak acids are the species on the left side of the table from HIO3 down to H2O.
Aqueous molecules of the conjugate acid and base coexist in solution.
→ Ex: HIO3(aq) ⇌ H+(aq) + IO3-(aq)The last two species on the left, OH- and NH3, cannot act as acids in aqueous solutions.
Weak BASES
Weak bases are the species on the right side of the table from H2O down to PO43-.
Aqueous molecules of the conjugate base and acid coexist in solution.
The top six species on the right, Br-, Cl-, I-, NO3-, ClO4-, and HSO4-, cannot act as bases in aqueous solutions.
Levelling effect
All strong acids and bases have IDENTICAL STRENGTHS because they are 100% ionized in aqueous solutions.
Strong acids and bases have a higher electrical conductivity because they ionize completely.
Six strong acids, HBr, HCl, HI, HNO3, HClO4, and H2SO4, cannot exist as molecules in aqueous solutions because they ionize completely to produce H3O+ and an anion.
→ H3O+ is the strongest acid that can exist in aqueous solution.

Equilibrium constant for the ionization of water
ACIDIC: [H3O+] > [OH-]
NEUTRAL: [H3O+] = [OH-]
BASIC: [H3O+] < [OH-]
SELF-IONIZATION of water can be represented as: energy + H2O + H2O ⇌ H3O+ + OH-
Since H2O is pure liquid, [H2O] is constant and can be ignored in the expression of the EQUILIBRIUM CONSTANT OF WATER, Kw:
Kw = [H3O+][OH-] = 1.00 × 10-14 (at 25°C)
Kw only varies with temperature change.
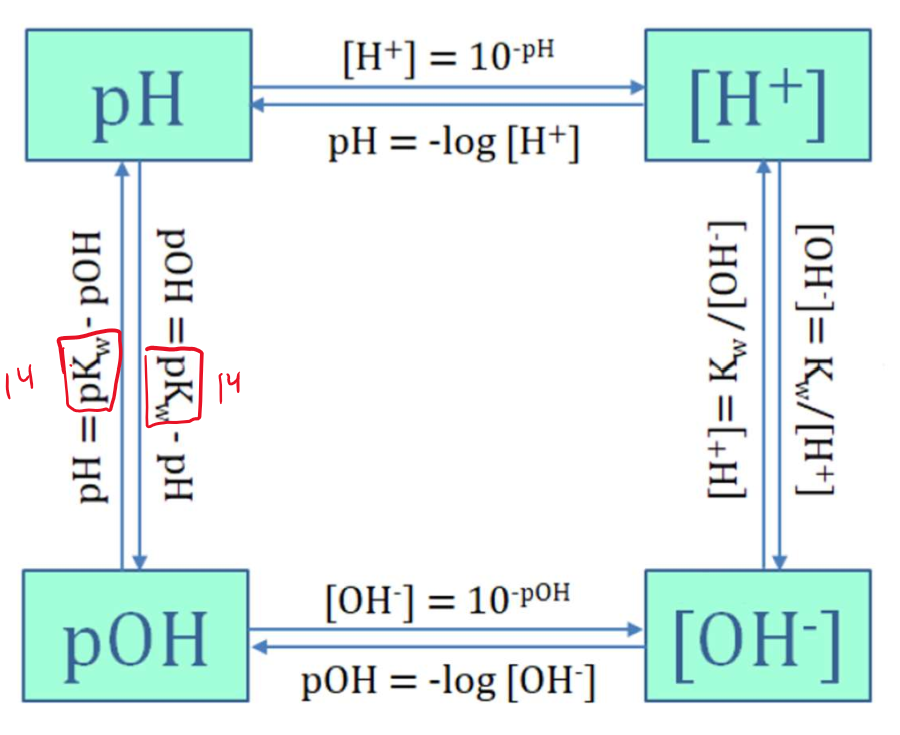
pH and pOH
pH of a solution is the negative logarithm of the molar concentration of hydronium ion (H3O+)
pH = - log [H3O+]
[H3O+] = antilog (-pH) = 10-pH
pOH is the negative logarithm of the molar concentration of hydroxide ion (OH-)
pH = - log [OH-]
[OH-] = antilog (-pOH) = 10-pOH
NOTE: For pH and pOH, only the numbers after the decimal point are significant.
pH + pOH = pKw = 14.00
pH < 7.00 or pOH > 7.00 → acidic solution
pH > 7.00 or pOH < 7.00 → basic solution
pH = pOH = 7.00 → neutral solution
Low pH and pOH values mean relatively high values of [H3O+] and [OH-], respectively.
High pH and pOH values mean relatively low values of [H3O+] and [OH-], respectively.
ACID IONIZATION CONSTANT
Written as Ka
The greater the value of Ka, the stronger the acid.
Large Ka value indicates that the equilibrium favours products, hence these acids are stronger because they have greater ionization.
The Ka values for STRONG acids are not listed in the table of Strengths of Acids since these acids are 100% ionized, and the concentration of the unionized acid in the denominator of the Ka expression is zero.
BASE IONIZATION CONSTANT
Written as Kb
The greater the Kb value, the stronger the base.
Kb values must be calculated using Ka values of the conjugate acids.
Relationship between Ka and Kb
Ka x Kb = Kw (at 25°C, Kw = 1.0 x 10-14)
Ka ∝ 1/Kb (Reciprocal nature between Ka and Kb)
The conjugate base of a weak acid is a “strong” base.
The conjugate base of a “strong” acid is a weak base.
→ The stronger the acid, the weaker the conjugate base.
The relative strengths of Acids and Bases
HCO3- + H2PO4- ⇌ H2CO3 + HPO42-
If solutions containing amphiprotic ions such as HCO3- and H2PO4- are mixed, the stronger of the two acids will donate a proton.
HCO3- (Ka = 5.6 × 10-11) and H2PO4- (Ka = 6.2 × 10-8). Since H2PO4- has a larger Ka, it will donate a proton to HCO3-.
There is a “proton competition” between the acids in the solution.
Since H2PO4- has a greater tendency to donate protons, it will be the stronger acid and the equilibrium will favour the products.
Hydrolysis
Reaction between water and the cation or anion (or both) contained in the salt to produce an acidic or basic solution.
SPECTATOR IONS do not hydrolyze and remain the same in the reaction.
SPECTATOR CATIONS (+): alkali metals (group 1) and alkaline earth metals (group 2)
SPECTATOR ANIONS (-): first five ions at the top right of the table of Relative Strengths of Acids (conjugate bases of strong acids): ClO4-, I-, Br-, Cl-, and NO3-
HSO4- is not a spectator ion since it is a weak acid.
Anionic (-) hydrolysis
Anions (-) hydrolyze to produce OH-(aq) and give a basic solution.
Act as BASES when reacting with water.
B-(aq) + H2O(l) ⇌ HB(aq) + OH-(aq)
Cationic (+) hydrolysis
Cations (+) hydrolyze to produce H3O+(aq) and give an acidic solution.
Act as ACIDS when reacting with water.
HA+(aq) + H2O(l) → A-(aq) + H3O+(aq)
Summary of Hydrolysis
STRONG ACID - STRONG BASE salts → NEUTRAL
STRONG ACID - weak base salts → ACIDIC
weak acid - STRONG BASE salts → BASIC
Metal vs. Nonmetal Oxides
METAL OXIDES (group 1 & 2 metals) produce BASIC solutions when dissolved in water (basic anhydrides).
Na2O(s) → 2Na+(aq) + O2-(aq)
O2-(aq) + H2O(l) → 2OH-(aq)
Na2O(s) + H2O(l) → 2NaOH(aq)
NONMETAL OXIDES produce ACIDIC solutions when dissolved in water (acidic anhydrides).
CO2(g) + H2O(l) → H2CO3(aq)
H2CO3(aq) + H2O(l) ⇌ HCO3-(aq) + H3O+(aq)